Omega-6
now browsing by category
The Hidden Key to Boundless Energy
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2025/04/06/the-hidden-key-to-boundless-energy.aspx
Analysis by Dr. Joseph Mercola April 06, 2025
STORY AT-A-GLANCE
- Modern environmental factors including seed oils, endocrine disruptors, estrogens and EMFs allow harmful gut bacteria to proliferate, producing endotoxins that severely compromise mitochondrial function and reduce cellular energy production
- While ketogenic diets provide short-term benefits, long-term carbohydrate restriction impairs mitochondrial function and creates reductive stress in cells, necessitating a more balanced approach
- Excessive consumption of omega-6-rich seed oils severely damages mitochondrial function and makes skin more susceptible to sun damage, making these processed oils one of the biggest threats to health
- A healthy gut environment requires proper cellular energy to maintain low oxygen levels, allowing beneficial bacteria to thrive and produce protective short-chain fatty acids that strengthen your intestinal barrier
- Restoring health requires systematically reducing exposure to environmental toxins while gradually reintroducing healthy carbohydrates to support mitochondrial function and maintain proper gut bacteria balance
In my interview with Sean Kim of Growth Minds, we discussed the decades I’ve spent searching for the best ways to help you reclaim your health.1 When you consider how different modern lifestyles are from our ancestors’ days, it reveals many clues about why you might feel tired, run-down or prone to illness. Those ancestors had their own health challenges, but they weren’t swimming in artificial chemicals, electromagnetic fields and processed seed oils that drive chronic diseases.
You face these threats every day, and your body is likely struggling as a result. I’ve devoted my life to understanding how food, environment and daily habits affect you at the cellular level. That journey led me to study how your mitochondria produce the energy you need. Mitochondria are known as your cells’ power stations.
They depend on proper fuel, stable hormone levels and minimal toxic exposures to keep you thriving. If those factors are off balance, you’ll feel it. The question is: how do you get them back on track?
While a ketogenic diet or intermittent fasting help you lose weight initially, they’re a short-term fix with long-term consequences. As I explained to Kim, there’s a deeper story about how your body responds to various fuels, especially when you’ve been under stress or exposed to toxic influences.
You have to look at your gut, your hormone systems and your environment to fully understand what’s going on and restore optimal health. When I first explored diets high in fat and extremely low in carbohydrates, I saw benefits for some people in specific circumstances. Over time, however, I discovered that your system needs more than a strict low-carb diet provides.
Rethinking What It Means to Eat Well
In my interview with Kim, I made it clear that I used to be a leading advocate of ketogenic diets. I even wrote a No. 1 bestselling book on the topic. Many people have used a ketogenic diet with good outcomes for weight loss and insulin control, and I believed that kind of diet could support you in turning your health around. The results people experienced weren’t imaginary. Many of them had real successes.
Over time, however, more detailed research into mitochondrial function made me change my stance. It’s not enough to measure your short-term results. You have to look at what happens over many years. If you keep forcing your body into a state of ultra-low carbohydrate intake, you risk backing up electron flow in your mitochondria. That jammed-up electron flow weakens your cells’ ability to produce steady energy, a phenomenon otherwise known as reductive stress.
It also encourages shifts in your gut bacteria that harm you more than help you. You need healthy gut bacteria to make short-chain fatty acids, which keep your colon lining strong and keep harmful pathogens in check. A balanced intake of healthy carbohydrates is key once you’ve corrected the root concerns. Your brain needs glucose, and while you can survive on fewer carbs for a while, it’s easy to slip into a stressful metabolic state if you don’t consume enough healthy carbs.
How Your Environment Shapes Your Health
Everyday toxins also affect you at the cellular level. Throughout our talk, I explained to Kim that I’ve identified four main stressors that diminish your mitochondrial energy production. These factors silently harm your gut health, disrupt your hormones and trigger damaging oxidative stress.
First, you have the overconsumption of omega-6 seed oils, which are rich in linoleic acid. These highly processed cooking oils are the single biggest nutritional danger you face. You’ll find them in countless packaged foods, snack items and restaurant meals. The main reason why excess LA causes disease is that it prevents your mitochondria from working well. It also makes sun exposure more damaging due to the accumulation of these fats in your skin cells.
Second, you have excess endocrine-disrupting chemicals (EDCs) in your environment. These come from plastics, personal care products and even certain pesticides and mimic hormones, like estrogen, in your body. Many of these endocrine-disrupting chemicals reduce fertility and create hormonal imbalances. Xenoestrogens found in everyday items like plastic are one example of EDCs with widespread reach.
It’s also important to minimize exposure to synthetic estrogens, such as those found in hormone replacement therapy and oral contraceptives. Estrogen increases intracellular calcium levels and decreases mitochondrial function. In fact, estrogen dominance is nearly as dangerous as excessive LA intake when it comes to destroying your mitochondrial function.
The third significant threat to cellular health comes from pervasive exposure to electromagnetic fields (EMFs) due to the proliferation of wireless technologies. EMFs increase calcium ion concentrations within cells, resulting in the production of harmful free radicals.
Together, widespread exposure to LA in seed oils, EDCs in plastics and EMFs impair your cells’ ability to generate energy efficiently. This energy deficit makes it challenging to sustain the oxygen-free gut environment necessary for beneficial bacteria to flourish.
As your gut barrier weakens, it allows harmful substances to breach your intestinal wall and enter your bloodstream. This intrusion triggers a systemic inflammatory response, with wide-ranging effects on your health. Of particular concern is the proliferation of oxygen-tolerant bacteria, which are not ideally suited for the gut environment.
These microorganisms produce a potent form of endotoxin — the fourth major threat to your cellular health — known as lipopolysaccharide (LPS). When LPS enters your bloodstream through a compromised gut barrier, it leads to a severe condition known as endotoxemia, which often progresses to septic shock — a state of systemic inflammation that’s sometimes fatal.

Save This Article for Later – Get the PDF Now
Restoring Gut Health as Your Foundation
A healthy gut is pivotal to your well-being. In my interview with Kim, I explained that if your healthy gut bacteria can’t thrive, your body faces one hurdle after another. An oxygen-free environment is necessary for beneficial bacteria that create short-chain fatty acids such as butyrate, propionate and acetate to thrive. These compounds help keep your colon lining strong by nourishing the cells that line your gut wall.
Your body needs cellular energy to keep oxygen levels low in your colon, however. So, if your mitochondria aren’t functioning properly and your cellular energy is low, you’re likely to have excess oxygen in your colon that boosts harmful bacteria.
The end result is an upsurge in toxic byproducts, including more potent forms of endotoxin. That’s why simply cutting carbohydrates might seem to help in the short term: if you starve harmful bacteria of their favorite fuels, they don’t multiply so fast.
Yet you pay for it later by ultimately decreasing the cellular energy you need for robust digestion and a healthy metabolism. A diet that includes high-quality fiber from vegetables and other sources of healthy carbohydrates is key, but if you have a compromised gut, it’s important to start with easier-to-digest options, like white rice or slowly sipping dextrose water daily for a week or two.
You want to steer clear of a low-carb diet, especially long term. If you keep your body in a constant energy deficit, you’re only compounding your mitochondrial problems. You’re also setting yourself up for increased stress hormone release, which breaks down your lean muscle tissue to make emergency glucose.
By cutting out mitochondrial poisons and nourishing your gut with healthy carbohydrates, you give your body the chance to restore that protective mucus layer, keep oxygen levels low in your colon and restore mitochondrial health for increased cellular energy.
When you remove the factors that destroy your cellular energy, you can then enjoy moderate to higher carbohydrate intake without wrecking your metabolic function. This might mean 200 to 350 grams of quality carbohydrates in a day, but the exact amount varies by your personal needs, activity level and genetics. The key is to focus on real, whole-food sources instead of ultraprocessed carbs that contain seed oils and refined sugar.
Let me emphasize once more that you should clear out the elements causing harm before you increase your carbohydrate intake. That means cutting back on omega-6-rich seed oils, limiting endocrine-disrupting chemicals, reducing EMF exposure and repairing your gut so it’s able to handle more fiber.
Practical Steps to Tame the Toxins
During my discussion with Kim, we touched on ways to reduce exposure to chemicals and stressors, so you enhance your health from the inside out. If you want to limit microplastics and hormone-disrupting substances, start by cutting down on plastic packaging.
Swap plastic containers for glass whenever possible, and avoid heating foods in plastic. Be mindful of personal care items with synthetic fragrances or complex chemical blends. Even so-called “organic” products often contain compounds that destabilize your hormones, so read labels carefully.
You also want to be wary of your Wi-Fi router and the constant signals from your phone. If you keep your phone by your bed at night, you’re exposing your body to nonstop EMFs. Turning off your wireless devices or switching to airplane mode gives your cells a break, but a better option is to turn off your Wi-Fi at night — or even shut off the power to your bedroom.
Also, try wired internet at home and see whether you notice improvements in your sleep or focus. As you move beyond eating well, also look into ways to speed up the removal of toxins. Sweating is one of the best methods. Traditional exercise does the job as you increase circulation, but an infrared sauna takes it further if you have access to one.
Grounding, or walking barefoot on natural surfaces like sand or soil, also helps reduce extra electrical charges in your body. You still want to watch out for walking barefoot on unnaturally hard floors every day, which promotes the development of joint or foot issues. Even so, a dose of nature is calming, and you might find that grounding on natural surfaces like grass or the ocean is a soothing method to connect with your environment.
During the interview, I also noted that sunlight is both beneficial and at times harmful, depending on your overall health. You absolutely need adequate sun exposure to help your body produce vitamin D and provide other benefits. However, if you’re carrying an excessive amount omega-6 seed oils in your skin cells, they’re prone to oxidation when exposed to sunlight, increasing the risk of skin damage.
Too many of these oxidizable fats in your tissues magnifies any negative effects from UV rays. To maximize the benefits of sun exposure and minimize the risks, eliminate seed oils from your diet. I recommend avoiding sun during peak hours (from 10 a.m. to 4 p.m. in most U.S. regions) until you’ve been seed-oil-free for at least six months.
The Promise of Future Health Innovations
As I told Kim, I believe technology itself becomes a friend if it’s harnessed in the right way. Yes, you should reduce EMF exposure from your phone and your Wi-Fi. Still, advanced computer systems, including artificial intelligence, help you monitor your health in real time.
In the near future, you might use AI-driven software that tracks your daily habits, recognizes patterns in your hormone levels and reminds you to make adjustments to your diet or supplement routine. It’s like having a health coach who’s always there, offering personalized feedback based on data from wearable devices or blood tests.
Progress in the field of mitochondrial research is also advancing at a rapid pace. We’ve come a long way in understanding how molecules like coenzyme Q10 help push electrons through your mitochondrial chain. Further investigations could pinpoint more specific strategies to optimize that electron flow, so you generate energy without building up damaging free radicals.
I’m particularly excited about new insights into gut therapies that restore the colon’s oxygen-free environment, such as an approach that combines targeted probiotics with supportive nutrients to revive the cells lining your gut.
Doing so would let beneficial microbes flourish and block harmful bacteria from expanding. This holds the promise of turning gut health into a more precise science, where you measure shifts in your microbiome composition and match specific interventions for faster results.
As these new approaches gain traction, I’m working to gather data and share it with you. I’m driven by a mission to show you that your body already has the blueprint for abundant energy and balanced hormones. The problem is interference. Environmental pollutants, seed oils and stressors have created roadblocks. If you reduce them systematically, you’ll give yourself a fresh start.
Charting Your Path to Lasting Vitality
In my interview with Kim, I emphasized that your mitochondria lie at the heart of your health story. They decide whether you have the energy to thrive or whether you struggle with chronic fatigue and cellular stress. By addressing the four main stressors — seed oils, endocrine-disrupting chemicals, endotoxins and EMFs — you free up your mitochondria to run at full power. You stop feeding the processes that tear down your gut and your energy.
You also open the door for a truly balanced diet, one that includes not just healthy fats and proteins, but also the right kind of carbohydrates. You deserve to feel vibrant, and your cells are programmed to help you get there.
Clear away plastic toxins, turn off your Wi-Fi at night, choose glass bottles and avoid consuming seed oils. As your gut health improves, introduce better fiber sources that feed your beneficial gut microbes and support mucin production, which protects you from leaky gut.
If you take these steps, you’ll likely see a positive ripple effect. Your thyroid might perk up, your hormones rebalance and your gut wall becomes sturdier. In time, you might even be able to tolerate moderate sun exposure without burning as easily, since your cell membranes are no longer packed with unhealthy fats.
No matter where you are in your health journey, let this knowledge empower you — you can fix the hidden obstacles that drain your energy and derail your gut, and feel confident in a plan that nourishes you from your cells outward, letting you enjoy a fuller life.
This is what I hope you’ll take away from my conversation with Kim: you have more control over your well-being than you realize. When you align your habits with what your body needs, you unleash the boundless energy that’s been waiting inside you all along.
Understanding the Root Causes of Dyslipidemia in Atherosclerotic Cardiovascular Disease
Reproduced from original OMNS article (OrthoMolecular News Service):
http://orthomolecular.org/
Subscribe to the free Orthomolecular Newsletter: http://orthomolecular.org/subscribe.html
Go to the OMNS Archive: http://orthomolecular.org/resources/omns/index.shtml
Orthomolecular Medicine News Service, January 10, 2025
Richard Z. Cheng, M.D., Ph.D., Thomas E. Levy, M.D., J.D.
Highlights
A paradigm shift from the cholesterol-centric focus on symptom management to addressing the root causes of ASCVD has demonstrated potential for prevention and reversal, as shown by our recently reported 10 ASCVD reversal cases (1).
Abstract
Dyslipidemia has long been the primary target for atherosclerotic cardiovascular disease (ASCVD) treatment. However, we have recently presented compelling evidence demonstrating that dyslipidemia is an intermediary mechanistic step, not a root cause of ASCVD, and that the American Heart Association’s decades-long cholesterol-centric dogma is both unreasonable and potentially unethical, bordering on criminal negligence (2).
In our international consultation services, we have shifted from this outdated paradigm to an orthomolecular medicine-based integrative approach, focusing on restoring biochemical balance (between nutrients and toxins) and physiological harmony (among various hormones). Using this approach, we recently reported a series of 10 successful ASCVD reversal cases (1).
This paper explores the multifactorial root causes contributing to dyslipidemia, including dietary factors, nutritional deficiencies, infections, physical inactivity, and hormonal imbalances. Special attention is given to the roles of high-carbohydrate diets, ultra-processed foods, seed oils (containing high amounts of omega-6 PUFA), and high-fructose consumption. The effects of micronutrient deficiencies, such as those of vitamins B, C, D, E, and magnesium, are examined in the context of lipid metabolism. Additionally, the paper highlights the impact of chronic infections, sedentary lifestyles, and hormonal dysregulation on lipid abnormalities.
Understanding these key root causes provides a foundation for more effective prevention and treatment strategies (3). In future papers, we plan to explore these topics in greater detail, advocating for a paradigm shift from cholesterol-centric management to addressing the underlying causes of dyslipidemia and ASCVD.
Introduction
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of morbidity and mortality worldwide. For decades, cholesterol and dyslipidemia have been central to ASCVD management strategies. However, our prior critiques of the cholesterol-centric paradigm have underscored that dyslipidemia is not the root cause but rather an intermediary mechanism of ASCV (2). Here we explore the multifactorial root causes underlying dyslipidemia, and advocate for prevention and treatment strategies that address these root causes. We focus here on categorizing the primary root causes contributing to ASCVD through dyslipidemia. More comprehensive discussions on these root causes will be presented where appropriate in subsequent papers in this series.
1. Dietary factors and dyslipidemia
- High-carbohydrate diets have been strongly associated with dyslipidemia, particularly characterized by increased triglycerides and decreased HDL cholesterol levels (4–6). This effect is especially pronounced with high glycemic index carbohydrates (5). The mechanism may involve reduced clearance of LDL particles and increased production of their precursors (7). Carbohydrate-induced hypertriglyceridemia occurs when dietary carbohydrate exceeds 55% of energy intake, despite reduced dietary fat (8). This paradoxical effect may be due to enhanced intestinal de novo lipogenesis and mobilization of stored lipids (9). However, the impact of carbohydrates on lipid metabolism is complex, with some studies suggesting that low-carbohydrate diets may have beneficial effects on atherogenic dyslipidemia (10).
- Low-carbohydrate ketogenic diets (KDs) have shown promising effects in improving metabolic disorders, particularly dyslipidemia. KDs can lead to significant reductions in triglycerides, total cholesterol, and LDL cholesterol, while increasing HDL cholesterol (11,12). These diets have been found to improve insulin sensitivity, reverse atherogenic dyslipidemia, and reduce inflammatory biomarkers associated with cardiovascular disease (13,14). KDs have also demonstrated benefits in managing obesity, metabolic syndrome, and type 2 diabetes (15,16). Studies have shown that KDs can decrease fasting serum insulin concentrations, improve LDL particle size, and reduce postprandial lipemia (11,12). While the optimal carbohydrate proportion and diet duration require further investigation, KDs appear to be a safe and effective approach for treating metabolic disorders (17,18).
- Ultra-processed foods and dyslipidemia. High consumption of ultra-processed foods (UPF) has been shown to be associated with an increased risk of dyslipidemia and other cardiometabolic disorders. Multiple prospective cohort studies have found that individuals with higher UPF intake have significantly greater odds of developing hypertriglyceridemia, low HDL cholesterol, and hypercholesterolemia (19,20). This association has been observed in both adults and adolescents (21,22). Systematic reviews and meta-analyses confirm these findings, reporting consistent positive associations between UPF consumption and increased risk of dyslipidemia, as well as diabetes, hypertension, and obesity (23,24). Longitudinal studies in children have also shown that higher UPF intake is associated with elevated total cholesterol and triglyceride levels (25). Proposed mechanisms include altered food matrix, toxicity from additives, and processing-induced contaminants affecting lipid metabolism, gut microbiota, and inflammatory pathways (26).
- Seed oils (rich in omega-6 PUFA) and dyslipidemia. Research suggests that high intake of omega-6 polyunsaturated fatty acids (PUFAs) from seed oils may contribute to inflammation, oxidative stress, and atherosclerosis (27). Despite recommendations for omega-6 PUFA consumption, some studies indicate potential long-term side effects, including hyperinsulinemia and increased cancer risk (28). Flaxseed and its oil, rich in omega-3 fatty acids, have demonstrated positive impacts on cardiovascular risk factors and dyslipidemia (29,30). Adjusting the omega-6 to omega-3 PUFA ratio may be crucial in managing chronic diseases (30). During cooking, both omega-3 and omega-6 high PUFA seed oils are readily oxidized, become rancid, and may produce harmful trans-fats (72).
- High fructose (found in HFCS and fruits). Research suggests that high fructose consumption, particularly from high-fructose corn syrup (HFCS), may contribute to dyslipidemia and other metabolic disorders. Studies have shown that fructose intake can increase postprandial triglycerides, LDL cholesterol, and apolipoprotein B levels (32,33). Fructose consumption has also been linked to visceral adiposity, insulin resistance, and hepatic de novo lipogenesis (fatty liver disease) (34,35). The metabolic effects of fructose differ from glucose due to its rapid hepatic conversion and extraction (36). While some studies found no significant metabolic differences between HFCS and sucrose (37), others suggest that HFCS consumption at 25% of energy requirements can increase cardiovascular disease risk factors comparably to fructose (32). Recent research emphasizes the synergistic effects of glucose and fructose on lipid metabolism, supporting public health efforts to reduce sugar intake (38,39).
2. Nutritional deficiency and dyslipidemia
Many vitamins and micronutrients play critical roles in lipid and energy metabolism, and deficiencies—whether isolated or combined—can lead to metabolic disturbances. Below are some key examples:
- B vitamins. Niacin and vitamin B6 have shown significant potential in managing dyslipidemia and associated cardiovascular risks. Niacin supplementation can lower triglycerides, LDL, and VLDL levels while increasing HDL (40). B vitamin supplementation improves lipid metabolism and reduces inflammation in patients with stable coronary artery disease (41). Animal studies have demonstrated antihyperlipidemic and hepatoprotective effects of vitamin B6 (42). Deficiencies in vitamins B6 and B12 are frequently reported in hyperlipidemic patient (43). Higher dietary niacin intake is associated with a reduced risk of dyslipidemia (44).
- Vitamin C and dyslipidemia. Research demonstrates that vitamin C supplementation can improve lipid profiles by lowering total cholesterol, LDL cholesterol, and triglycerides, particularly in individuals with hypercholesterolemia or diabetes (45–47). Some studies also report increases in HDL cholesterol (48,49). Beneficial effects of vitamin C have been observed across diverse groups, including diabetics, hemodialysis patients, and oil workers exposed to petroleum fumes (50,51). A meta-analysis of 13 randomized controlled trials confirmed that vitamin C supplementation significantly reduces LDL cholesterol and triglycerides in hypercholesterolemic individuals (46). The effects of vitamin C vary depending on dosage, duration, and individual health status (47). Dr. Linus Pauling’s pioneering work on vitamin C and cardiovascular disease laid the foundation for understanding its role in vascular health, indirectly linking it to lipid metabolism. We plan to dedicate a paper to further explore Pauling’s insights and their relevance to dyslipidemia and ASCVD. One of us (TEL) discusses vitamin C’s role in improving lipid profiles, combating oxidative stress, and supporting vascular health in the books Primal Panacea (52) and Stop America’s Number One Killer (53).
- Vitamin D and dyslipidemia. Vitamin D deficiency is significantly associated with dyslipidemia. Studies reveal that individuals with lower serum 25-hydroxyvitamin D levels are more likely to exhibit abnormal lipid profiles, including elevated total cholesterol, LDL, and triglycerides, and decreased HDL (54–57). This relationship persists even after adjusting for confounding factors. Vitamin D deficiency is linked to alterations in metabolomic profiles, particularly sphingolipid pathway (58). Interactions with other micronutrients, such as vitamin A, zinc, and magnesium, may influence vitamin D’s impact on lipid metabolism (59). Our recent comprehensive review of vitamin D demonstrates that maintaining optimal serum levels above 40 ng/mL reduces the risk of cardiovascular disease incidence and mortality (60) (accepted for publication by Nutrients).
- Vitamin E and dyslipidemia. Vitamin E has shown anti-atherosclerotic properties (61). Research on vitamin E and dyslipidemia shows mixed results. Some studies suggest that vitamin E supplementation can improve lipid profiles in dyslipidemic patients, reducing total cholesterol, LDL-C, and triglycerides (62,63). Higher serum vitamin E levels have been associated with a more favorable lipid profile (64). Vitamin E supplementation has been shown to suppress elevated plasma lipid peroxides and increase serum antioxidant activity (65). The impact of antioxidative vitamins on lipid profiles varies based on dosage, duration, and individual health status (47).
- Magnesium and dyslipidemia. Hypomagnesemia has been linked to metabolic abnormalities and dyslipidemia (66–70). Studies report negative correlations between serum magnesium and triglycerides, LDL, and total cholesterol, while positive correlations are observed with HDL cholesterol (70,71).
3. Infections and dyslipidemia
- Infections promote dyslipidemia. Dyslipidemia is a common complication in HIV-infected patients and those with COVID-19, associated with increased severity and mortality (72). It is characterized by elevated total cholesterol, LDL, and triglycerides, with decreased HDL (73,74). The pathogenesis involves inflammation, oxidative stress, and lipid peroxidation (75). These lipid abnormalities may increase cardiovascular risk in HIV patients (76,77). Research suggests a significant association between oral infections, particularly periodontitis, and systemic metabolic disturbances. Periodontitis has been linked to increased risk of cardiovascular diseases and dyslipidemia (78,79). Studies have found higher levels of total cholesterol, LDL cholesterol, and triglycerides, along with lower HDL cholesterol, in individuals with periodontitis (80,81). Chronic oral infection with Porphyromonas gingivalis, a key periodontal pathogen, has been shown to accelerate atheroma formation by altering lipid profiles in animal models (82). The relationship between periodontitis and hyperlipidemia appears bidirectional, with elevated triglycerides potentially modulating inflammatory responses to periodontal pathogens (83). The underlying mechanisms involve systemic inflammation, metabolic endotoxemia, and genetic factors that influence both oral infections and cardiometabolic diseases (84). These findings highlight the complex interplay between oral health and systemic metabolism.
- Infection control improves dyslipidemia. Periodontal treatment has been shown to improve lipid control (85). Eradication of Helicobacter pylori infection may decrease the risk of dyslipidemia (86).
4. Physical inactivity and dyslipidemia/high cholesterol
Research consistently shows an inverse relationship between physical activity (PA) and dyslipidemia. Higher PA levels are associated with increased HDL-C and decreased triglycerides in both men and women (87,88). Sedentary behavior increases the risk of dyslipidemia, while moderate-to-vigorous PA (MVPA) may reduce this risk (89,90). The prevalence of dyslipidemia is high in some populations, with limited awareness and treatment (91). Individuals meeting PA guidelines have lower odds of dyslipidemia, even with poor diet quality (91). However, adults with hypercholesterolemia are less likely to meet PA recommendations compared to those without (92). PA patterns, including timing and intensity, may influence lipid profiles differently (90). Overall, habitual PA is associated with more favorable lipid profiles and reduced cardiovascular disease risk (93,94).
5. Hormonal imbalance and dyslipidemia/high cholesterol
- Thyroid dysfunction, particularly hypothyroidism, is strongly associated with dyslipidemia and increased cardiovascular risk (95,96). Both overt and subclinical hypothyroidism can lead to elevated total cholesterol, LDL cholesterol, and apolipoprotein B levels, while potentially affecting HDL cholesterol and triglycerides (97,98). These lipid abnormalities are primarily due to reduced LDL receptor activity and altered regulation of cholesterol biosynthesis (99). Thyroid hormone replacement therapy has been shown to improve lipid profiles in overt hypothyroidism, but its benefits in subclinical hypothyroidism remain debated (99,100). Recent studies have also highlighted the role of thyroid hormones in regulating HDL function and cholesterol efflux (98). Given the prevalence of thyroid dysfunction and its impact on lipid metabolism, screening for thyroid disorders is recommended in patients with dyslipidemia (101).
- Cortisol imbalance significantly contributes to dyslipidemia, high cholesterol, and increased cardiovascular risk. Excess cortisol, such as in Cushing’s syndrome, is associated with elevated triglycerides, total cholesterol, and LDL cholesterol levels (102). Similarly, stress-induced cortisol elevation disrupts lipid metabolism, promoting atherogenesis and increasing the risk of atherosclerosis (103). Conversely, patients with metabolic syndrome and low cortisol levels exhibit less pronounced lipid disturbances (104). Elevated basal cortisol levels and reduced circadian variability have been linked to unfavorable lipid profiles, particularly in individuals with depressive and anxiety disorders (105). Additionally, the cortisol-to-DHEA ratio has been positively correlated with atherogenic lipid profiles in HIV patients with lipodystrophy (106). Glucocorticoid therapy, a common cause of cortisol excess, can lead to dyslipidemia and hypertension, further heightening cardiovascular disease risk (107). Excess cortisol is also strongly associated with obesity, hypertension, and metabolic syndrome (108,109). Furthermore, studies have found that elevated long-term cortisol levels, as measured in scalp hair, are linked to a history of cardiovascular disease (110). In obesity, higher cortisol concentrations are directly correlated with an increased risk of cardiovascular comorbidities (111). These findings highlight the multifaceted role of cortisol in dyslipidemia and emphasize the need to manage cortisol levels to mitigate cardiovascular risks effectively.
- Estrogen imbalance significantly impacts lipid metabolism and cholesterol levels. During menopause, estrogen deficiency leads to increased total cholesterol, LDL cholesterol, and triglycerides, while decreasing HDL cholesterol (112). High maternal estradiol levels can induce dyslipidemia in newborns by upregulating HMGCR expression in fetal hepatocytes (113). Estrogen administration in premenopausal women increases VLDL and HDL constituents, enhancing VLDL apoB and HDL apoA-I production (114). In postmenopausal women, estrogen therapy lowers LDL cholesterol levels (115). Estrogen treatment in cholesterol-fed rabbits attenuates atherosclerosis development by modulating lipoprotein metabolism (116,117). Endogenous sex hormones play a role in regulating lipid metabolism in postmenopausal women, with SHBG associated with a more favorable lipid profile (118). Estrogen administration in postmenopausal women decreases LDL cholesterol and hepatic triglyceride lipase activity while increasing HDL cholesterol (119).
- Progesterone imbalance can significantly impact lipid metabolism and cholesterol levels. Progesterone administration in rats led to increased hepatic triglycerides and cholesterol esters, while decreasing plasma cholesterol levels (120). In cultured cells, progesterone inhibited cholesterol biosynthesis (121). Dyslipidemia affected ovarian steroidogenesis in mice through oxidative stress, inflammation, and insulin resistance (122). In premenopausal women, ovarian lipid metabolism influenced circulating lipids (123). Estrogen plus progesterone replacement therapy in postmenopausal women lowered lipoprotein[a] levels and improved overall lipid profiles (124). High-dose medroxyprogesterone decreased total, LDL, and HDL cholesterol in postmenopausal women (125). In children, progesterone/estradiol ratios were associated with LDL-cholesterol levels (126). Female runners with menstrual irregularities showed altered steroid hormone and lipid profiles compared to eumenorrheic counterparts (127).
- Testosterone imbalance can significantly impact lipid metabolism and cholesterol levels. Research suggests a complex relationship between testosterone and lipid profiles. Low testosterone levels are associated with adverse lipid profiles, including higher total cholesterol and triglycerides, and lower high-density lipoprotein (HDL) cholesterol (128,129). Conversely, higher testosterone levels correlate with increased HDL cholesterol in men, particularly those with cardiovascular disease (130,131). Testosterone deficiency may contribute to hypercholesterolemia through altered expression of hepatic PCSK9 and LDL receptors (132). The effect of testosterone on lipids varies with age, gender, race/ethnicity, and menopausal status (133). Exogenous testosterone administration in hypogonadal men may improve lipid profiles by decreasing LDL and total cholesterol, although it may also decrease HDL cholesterol (134). While testosterone’s influence on lipids is evident, its overall impact on cardiovascular disease risk remains unclear and requires further investigation (134,135).
Conclusion
Dyslipidemia, long regarded as a primary target in ASCVD management, is increasingly understood as an outcome of complex, multifactorial root causes. These root causes include dietary factors, such as high-carbohydrate diets, ultra-processed foods, seed oils, and high-fructose consumption, which significantly influence lipid metabolism. Nutritional deficiencies, including vitamins B, C, D, and E, and magnesium, further exacerbate dyslipidemia, while chronic infections and physical inactivity compound cardiovascular risk. Hormonal imbalances, including dysfunctions in thyroid hormones, estrogen, progesterone, testosterone, and cortisol, also play a pivotal role in lipid abnormalities.
Addressing these underlying factors presents an opportunity to move beyond the traditional cholesterol-centric paradigm. Strategies such as dietary modifications, increased physical activity, infection control, and optimization of nutritional and hormonal balance can significantly improve lipid profiles, reduce cardiovascular risk, and even reverse ASCVD in some cases, as we have demonstrated in our recent report (1).
By focusing on the root causes of dyslipidemia, healthcare providers can offer more personalized and effective interventions, shifting the emphasis from symptom management to true disease prevention and reversal. This approach has the potential to improve not only ASCVD outcomes but also overall cardiovascular health and longevity. Future studies should prioritize the integration of these multifaceted strategies into clinical practice, emphasizing the importance of addressing the root causes of dyslipidemia for sustainable cardiovascular health.
References:
1. Cheng RZ, Duan L, Levy TE. A Holistic Approach to ASCVD: Summary of a Novel Framework and Report of 10 Case Studies. Orthomol Med News Serv [Internet]. 2024 Nov 27;20(20). Available from: https://orthomolecular.org/resources/omns/v20n20.shtml
2. Cheng RZ, Levy TE. The Mismanagement of ASCVD: A Call for Root Cause Solutions Beyond Cholesterol. Orthomol Med News Serv [Internet]. 2025 Jan 2 [cited 2025 Jan 5]; Available from: https://orthomolecular.activehosted.com/index.php?action=social&chash=0bb4aec1710521c12ee76289d9440817.345
3. Cheng RZ. Integrative Orthomolecular Medicine Protocol for ASCVD [Internet]. 2024. Available from: https://www.drwlc.com/blog/2024/08/01/integrative-orthomolecular-medicine-protocol-for-ascvd/
4. Polacow VO, Lancha Junior AH. [High-carbohydrate diets: effects on lipid metabolism, body adiposity and its association with physical activity and cardiovascular disease risk]. Arq Bras Endocrinol Metabol. 2007 Apr;51(3):389–400.
5. Shin WK, Shin S, Lee Jo koo. Carbohydrate Intake and Hyperlipidemia among Population with High‐Carbohydrate Diets: The Health Examinees Gem Study – Shin – 2021 – Molecular Nutrition & Food Research – Wiley Online Library. Mol Nutr Food Res [Internet]. [cited 2024 Dec 29]; Available from: https://onlinelibrary.wiley.com/doi/10.1002/mnfr.202000379
6. Jackson RL, Yates MT, McNerney CA, Kashyap ML. Diet and HDL Metabolism: High Carbohydrate vs. High Fat Diets. In: Malmendier CL, Alaupovic P, editors. Lipoproteins and Atherosclerosis [Internet]. Boston, MA: Springer US; 1987 [cited 2024 Nov 5]. p. 165–72. Available from: https://doi.org/10.1007/978-1-4684-1268-0_24
7. Houttu V, Grefhorst A, Cohn DM, Levels JHM, Roeters van Lennep J, Stroes ESG, et al. Severe Dyslipidemia Mimicking Familial Hypercholesterolemia Induced by High-Fat, Low-Carbohydrate Diets: A Critical Review. Nutrients. 2023 Feb 15;15(4):962.
8. Parks EJ. Effect of dietary carbohydrate on triglyceride metabolism in humans. J Nutr. 2001 Oct;131(10):2772S-2774S.
9. Stahel P, Xiao C, Lewis GF. Control of intestinal lipoprotein secretion by dietary carbohydrates. Curr Opin Lipidol. 2018 Feb;29(1):24–9.
10. Musunuru K. Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Lipids. 2010 Oct;45(10):907–14.
11. Sharman MJ, Kraemer WJ, Love DM, Avery NG, Gómez AL, Scheett TP, et al. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J Nutr. 2002 Jul;132(7):1879–85.
12. Hickey JT, Hickey L, Yancy WS, Hepburn J, Westman EC. Clinical use of a carbohydrate-restricted diet to treat the dyslipidemia of the metabolic syndrome. Metab Syndr Relat Disord. 2003 Sep;1(3):227–32.
13. O’Neill BJ. Effect of low-carbohydrate diets on cardiometabolic risk, insulin resistance, and metabolic syndrome. Curr Opin Endocrinol Diabetes Obes. 2020 Oct;27(5):301–7.
14. Zhang W, Guo X, Chen L, Chen T, Yu J, Wu C, et al. Ketogenic Diets and Cardio-Metabolic Diseases. Front Endocrinol. 2021;12:753039.
15. Moreno-Sepúlveda J, Capponi M. [The impact on metabolic and reproductive diseases of low-carbohydrate and ketogenic diets]. Rev Med Chil. 2020 Nov;148(11):1630–9.
16. Sakr HF, Sirasanagandla SR, Das S, Bima AI, Elsamanoudy AZ. Low-Carbohydrate Ketogenic Diet for Improvement of Glycemic Control: Mechanism of Action of Ketosis and Beneficial Effects. Curr Diabetes Rev. 2023;19(2):e110522204580.
17. Charlot A, Zoll J. Beneficial Effects of the Ketogenic Diet in Metabolic Syndrome: A Systematic Review. Diabetology. 2022 Apr 24;3(2):292–309.
18. Kayode TO, Rotimi ED, Afolayan AO, Kayode AAA. Ketogenic diet: A nutritional remedy for some metabolic disorders. J Educ Health Sport. 2020 Aug 10;10(8):180–8.
19. Donat-Vargas C, Sandoval-Insausti H, Rey-García J, Moreno-Franco B, Åkesson A, Banegas JR, et al. High Consumption of Ultra-Processed Food is Associated with Incident Dyslipidemia: A Prospective Study of Older Adults. J Nutr. 2021 Aug 7;151(8):2390–8.
20. Scaranni P de O da S, de Oliveira Cardoso L, Griep RH, Lotufo PA, Barreto SM, da Fonseca M de JM. Consumption of ultra-processed foods and incidence of dyslipidemias: the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Br J Nutr. 2022 Apr 22;1–22.
21. Lima LR, Nascimento LM, Gomes KRO, Martins M do C de CE, Rodrigues MTP, Frota K de MG. [Association between ultra-processed food consumption and lipid parameters among adolescents]. Cienc Saude Coletiva. 2020 Oct;25(10):4055–64.
22. Beserra JB, Soares NI da S, Marreiros CS, Carvalho CMRG de, Martins M do C de CE, Freitas B de JES de A, et al. [Do children and adolescents who consume ultra-processed foods have a worse lipid profile? A systematic review]. Cienc Saude Coletiva. 2020 Dec;25(12):4979–89.
23. Vitale M, Costabile G, Testa R, D’Abbronzo G, Nettore IC, Macchia PE, et al. Ultra-Processed Foods and Human Health: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Adv Nutr Bethesda Md. 2024 Jan;15(1):100121.
24. Mambrini SP, Menichetti F, Ravella S, Pellizzari M, De Amicis R, Foppiani A, et al. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients. 2023 May 31;15(11):2583.
25. Leffa PS, Hoffman DJ, Rauber F, Sangalli CN, Valmórbida JL, Vitolo MR. Longitudinal associations between ultra-processed foods and blood lipids in childhood. Br J Nutr. 2020 Aug 14;124(3):341–8.
26. Juul F, Vaidean G, Lin Y, Deierlein AL, Parekh N. Ultra-Processed Foods and Incident Cardiovascular Disease in the Framingham Offspring Study. J Am Coll Cardiol. 2021 Mar 30;77(12):1520–31.
27. DiNicolantonio JJ, O’Keefe J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Autoimmune Diseases, Asthma, and Allergies. Mo Med. 2021;118(5):453–9.
28. Yam D, Eliraz A, Berry EM. Diet and disease–the Israeli paradox: possible dangers of a high omega-6 polyunsaturated fatty acid diet. Isr J Med Sci. 1996 Nov;32(11):1134–43.
29. Vashishtha V, Barhwal K, Kumar A, Hota SK, Chaurasia OP, Kumar B. Effect of seabuckthorn seed oil in reducing cardiovascular risk factors: A longitudinal controlled trial on hypertensive subjects. Clin Nutr Edinb Scotl. 2017 Oct;36(5):1231–8.
30. Fawzy M, Nagi HM, Mourad R. BENEFICIAL EFFECT OF FLAXSEED AND FLAXSEED OIL BY ADJUSTING OMEGA6:OMEGA3 RATIO ON LIPID METABOLISM IN HIGH CHOLESTEROL DIET FED RATS. J Spec Educ Res. 2020 Apr 1;2020(58):117–42.
31. Obi J, Sakamoto T, Furihata K, Sato S, Honda M. Vegetables containing sulfur compounds promote trans-isomerization of unsaturated fatty acids in triacylglycerols during the cooking process. Food Res Int. 2025 Jan 1;200:115425.
32. Stanhope KL, Bremer AA, Medici V, Nakajima K, Ito Y, Nakano T, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011 Oct;96(10):E1596-1605.
33. Stanhope KL, Medici V, Bremer AA, Lee V, Lam HD, Nunez MV, et al. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am J Clin Nutr. 2015 Jun;101(6):1144–54.
34. Stanhope KL, Havel PJ. Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol. 2008 Feb;19(1):16–24.
35. Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010 Jan;90(1):23–46.
36. Schaefer EJ, Gleason JA, Dansinger ML. Dietary fructose and glucose differentially affect lipid and glucose homeostasis. J Nutr. 2009 Jun;139(6):1257S-1262S.
37. Angelopoulos TJ, Lowndes J, Zukley L, Melanson KJ, Nguyen V, Huffman A, et al. The effect of high-fructose corn syrup consumption on triglycerides and uric acid. J Nutr. 2009 Jun;139(6):1242S-1245S.
38. Gugliucci A. Sugar and Dyslipidemia: A Double-Hit, Perfect Storm. J Clin Med. 2023 Aug 31;12(17):5660.
39. Stanhope KL. Role of fructose-containing sugars in the epidemics of obesity and metabolic syndrome. Annu Rev Med. 2012;63:329–43.
40. Dayi T, Hoca M. Niasin Dislipidemi Riskini Azaltmada Potansiyel Bir Ajan Mıdır? İstanbul Gelişim Üniversitesi Sağlık Bilim Derg. 2022 Aug 29;(17):626–35.
41. Liu M, Wang Z, Liu S, Liu Y, Ma Y, Liu Y, et al. Effect of B vitamins supplementation on cardio-metabolic factors in patients with stable coronary artery disease: A randomized double-blind trial. Asia Pac J Clin Nutr. 2020;29(2):245–52.
42. Zhang Q, Zhang DL, Zhou XL, Li Q, He N, Zhang J, et al. Antihyperlipidemic and Hepatoprotective Properties of Vitamin B6 Supplementation in Rats with High-Fat Diet-Induced Hyperlipidemia. Endocr Metab Immune Disord Drug Targets. 2021;21(12):2260–72.
43. Al-Qusous MN, Al Madanat WKJ, Mohamed Hussein R. Association of Vitamins D, B6, and B12 Deficiencies with Hyperlipidemia Among Jordanian Adults. Rep Biochem Mol Biol. 2023 Oct;12(3):415–24.
44. Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys. 1955 Feb;54(2):558–9.
45. Chaudhari HV, Dakhale GN, Chaudhari S, Kolhe S, Hiware S, Mahatme M. The beneficial effec of vitamin C suppllemtation on serum lipids in type 2 diabetic patients: a randomized double blind study. Int J Diabetes Metab. 2012;20(2):53–8.
46. McRae MP. Vitamin C supplementation lowers serum low-density lipoprotein cholesterol and triglycerides: a meta-analysis of 13 randomized controlled trials. J Chiropr Med. 2008 Jun;7(2):48–58.
47. Mohseni S, Tabatabaei-Malazy O, Shadman Z, Khashayar P, Mohajeri-Tehrani M, Larijani B. Targeting dyslipidemia with antioxidative vitamins C, D, and E; a systematic review of meta-analysis studies. J Diabetes Metab Disord. 2021 Oct 21;20(2):2037–47.
48. Ness AR, Khaw KT, Bingham S, Day NE. Vitamin C status and serum lipids. Eur J Clin Nutr. 1996 Nov;50(11):724–9.
49. Cerná O, Ramacsay L, Ginter E. Plasma lipids, lipoproteins and atherogenic index in men and women administered vitamin C. Cor Vasa. 1992;34(3):246–54.
50. El Mashad GM, ElSayed HM, Nosair NA. Effect of vitamin C supplementation on lipid profile, serum uric acid, and ascorbic acid in children on hemodialysis. Saudi J Kidney Dis Transplant Off Publ Saudi Cent Organ Transplant Saudi Arab. 2016;27(6):1148–54.
51. George-Opuda IM, Etuk EJ, Elechi-Amadi KN, Okolonkwo BN, Adegoke OA, Ohaka TP, et al. Vitamin C Supplementation Lowered Atherogenic Lipid Parameters among Oil and Gas Workers Occupationally Exposed to Petroleum Fumes in Port Harcourt, Rivers State, Nigeria. J Adv Med Pharm Sci. 2024 Feb 19;26(3):45–52.
52. Levy TE, Gordon G. Primal Panacea. 2012 Second Printing edition. Henderson, NV: Medfox Publishing; 2011. 352 p.
53. Levy TE. Stop America’s #1 Killer: MD JD Levy, MD Julian Whitaker: 9780977952007: Amazon.com: Gateway [Internet]. [cited 2019 Jul 6]. Available from: https://www.amazon.com/Stop-Americas-Killer-MD-Levy/dp/0977952002/ref=sr_1_1?crid=2GE3D8VO3QMJL&keywords=stop+america+s+%231+killer&qid=1562416934&s=gateway&sprefix=stop+america%2Caps%2C428&sr=8-1
54. Sharba ZF, Shareef RH, Abd BA, Hameed EN. Association between Dyslipidemia and Vitamin D Deficiency: a Cross-Sectional Study. Folia Med (Plovdiv). 2021 Dec 31;63(6):965–9.
55. Chaudhuri JR, Mridula KR, Anamika A, Boddu DB, Misra PK, Lingaiah A, et al. Deficiency of 25-Hydroxyvitamin D and Dyslipidemia in Indian Subjects. J Lipids. 2013;2013:1–7.
56. Jiang X, Peng M, Chen S, Wu S, Zhang W. Vitamin D deficiency is associated with dyslipidemia: a cross-sectional study in 3788 subjects. Curr Med Res Opin. 2019 Jun 3;35(6):1059–63.
57. Doddamani DS, Shetty DP. The Association between Vitamin D Deficiency and Dyslipidemia. In 2020 [cited 2025 Jan 5]. Available from: https://www.semanticscholar.org/paper/The-Association-between-Vitamin-D-Deficiency-and-Doddamani-Shetty/50aa5e70d0f7a9edc9d72c5c7a8b0af5fca58866
58. Mousa H, Elrayess MA, Diboun I, Jackson SK, Zughaier SM. Metabolomics Profiling of Vitamin D Status in Relation to Dyslipidemia. Metabolites. 2022 Aug 22;12(8):771.
59. Khosravi-Boroujeni H, Ahmed F, Sarrafzadegan N. Is the Association between Vitamin D and Metabolic Syndrome Independent of Other Micronutrients? Int J Vitam Nutr Res Int Z Vitam- Ernahrungsforschung J Int Vitaminol Nutr. 2015 Dec;85(5–6):245–60.
60. Grant WB, Wimalawansa SJ, Pludowski P, Cheng RZ. Vitamin D: Evidence-Based Health Benefits and Recommendations for Population Guidelines. Nutrients [Internet]. Available from: www.mdpi.com/journal/nutrients
61. Saggini A, Anogeianaki A, Angelucci D, Cianchetti E, D’Alessandro M, Maccauro G, et al. Cholesterol and vitamins: revisited study. J Biol Regul Homeost Agents. 2011;25(4):505–15.
62. Vasanthi B, Kalaimathi B. Therapeutic Effect of Vitamin E in Patients with Dyslipidaemia. In 2012 [cited 2025 Jan 5]. Available from: https://www.semanticscholar.org/paper/Therapeutic-Effect-of-Vitamin-E-in-Patients-with-Vasanthi-Kalaimathi/2856f54306f952ff20d346526b46f31e4b462e23
63. Manimegalai R, Geetha A, Rajalakshmi K. Effect of vitamin-E on high fat diet induced hyperlipidemia in rats. Indian J Exp Biol. 1993 Aug;31(8):704–7.
64. Barzegar-Amini M, Ghazizadeh H, Seyedi SMR, Sadeghnia HR, Mohammadi A, Hassanzade-Daloee M, et al. Serum vitamin E as a significant prognostic factor in patients with dyslipidemia disorders. Diabetes Metab Syndr. 2019;13(1):666–71.
65. Szczeklik A, Gryglewski RJ, Domagala B, Dworski R, Basista M. Dietary supplementation with vitamin E in hyperlipoproteinemias: effects on plasma lipid peroxides, antioxidant activity, prostacyclin generation and platelet aggregability. Thromb Haemost. 1985 Aug 30;54(2):425–30.
66. Guerrero-Romero F, Rodríguez-Morán M. Magnesium and dyslipidemia [Internet]. 1st Edition. CRC Press; 2019 [cited 2025 Jan 5]. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9780429029141-5/magnesium-dyslipidemia-fernando-guerrero-romero-martha-rodr%C3%ADguez-mor%C3%A1n
67. Levy T. Magnesium: Reversing Disease: Levy MD, Jd: 9780998312408: Amazon.com: Books [Internet]. 2019 [cited 2022 Feb 12]. Available from: https://www.amazon.com/Magnesium-Reversing-MD-Jd-Levy/dp/0998312401/ref=pd_lpo_2?pd_rd_i=0998312401&psc=1
68. Dean C. The Magnesium Miracle (Second Edition): Dean M.D. N.D., Carolyn: 9780399594441: Amazon.com: Books [Internet]. 2017 [cited 2022 Feb 12]. Available from: https://www.amazon.com/Magnesium-Miracle-Second-Carolyn-Dean/dp/0399594442
69. Mishra S, Padmanaban P, Deepti G, G.Sarkar, Sumathi S, Toora BD. Serum Magnesium and Dyslipidemia in Type-2 Diabetes Mellitus. Biomed Res-Tokyo [Internet]. 2012 [cited 2025 Jan 5]; Available from: https://www.semanticscholar.org/paper/Serum-Magnesium-and-Dyslipidemia-in-Type-2-Diabetes-Mishra-Padmanaban/8d23a2bd9017cb57bb6ddda98789ba81c176b53c
70. Sajjan N, Shamsuddin M. A study of serum magnesium and dyslipidemia in type 2 diabetes mellitus patients. Int J Clin Biochem Res. 2016;3(1):36.
71. Deepti R, Nalini G, Anbazhagan. RELATIONSHIP BETWEEN HYPOMAGNESEMIA AND DYSLIPIDEMIA IN TYPE 2 DIABETES MELLITUS. Asian J Pharm Res Health Care [Internet]. 2014 Jul 1 [cited 2025 Jan 5]; Available from: https://www.semanticscholar.org/paper/RELATIONSHIP-BETWEEN-HYPOMAGNESEMIA-AND-IN-TYPE-2-Deepti-Nalini/5fd9c00eacce8aa45f93c3e5ea0961969ec3223b
72. Hariyanto TI, Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1463–5.
73. Lo J. Dyslipidemia and lipid management in HIV-infected patients. Curr Opin Endocrinol Diabetes Obes [Internet]. 2011 Apr [cited 2024 Dec 29];18(2). Available from: https://journals.lww.com/co-endocrinology/abstract/2011/04000/dyslipidemia_and_lipid_management_in_hiv_infected.9.aspx
74. Green ML. Evaluation and management of dyslipidemia in patients with HIV infection. J Gen Intern Med. 2002 Oct 1;17(10):797–810.
75.Feingold KR, Grunfeld C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000 [cited 2024 Dec 29]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK326741/
76. Kulasekaram R, Peters BS, Wierzbicki AS. Dyslipidaemia and cardiovascular risk in HIV infection. Curr Med Res Opin. 2005 Nov;21(11):1717–25.
77. Kotler DP. HIV and Antiretroviral Therapy: Lipid Abnormalities and Associated Cardiovascular Risk in HIV-Infected Patients. JAIDS J Acquir Immune Defic Syndr. Sept. 1, 20008;49:S79–85.
78. Mattila KJ, Pussinen PJ, Paju S. Dental infections and cardiovascular diseases: a review. J Periodontol. 2005 Nov;76(11 Suppl):2085–8.
79. Ma W, Zou Z, Yang L, Lin D, Guo J, Shan Z, et al. Exploring the bi-directional relationship between periodontitis and dyslipidemia: a comprehensive systematic review and meta-analysis. BMC Oral Health. 2024 Apr 29;24(1):508.
80. Moeintaghavi A, Haerian-Ardakani A, Talebi-Ardakani M, Tabatabaie I. Hyperlipidemia in patients with periodontitis. J Contemp Dent Pract. 2005 Aug 15;6(3):78–85.
81. Nibali L, D’Aiuto F, Griffiths G, Patel K, Suvan J, Tonetti MS. Severe periodontitis is associated with systemic inflammation and a dysmetabolic status: a case-control study. J Clin Periodontol. 2007 Nov;34(11):931–7.
82. Maekawa T, Takahashi N, Tabeta K, Aoki Y, Miyashita H, Miyauchi S, et al. Chronic Oral Infection with Porphyromonas gingivalis Accelerates Atheroma Formation by Shifting the Lipid Profile. Cardona PJ, editor. PLoS ONE. 2011 May 19;6(5):e20240.
83. Cutler CW, Shinedling EA, Nunn M, Jotwani R, Kim BO, Nares S, et al. Association between periodontitis and hyperlipidemia: cause or effect? J Periodontol. 1999 Dec;70(12):1429–34.
84. Janket SJ, Javaheri H, Ackerson LK, Ayilavarapu S, Meurman JH. Oral Infections, Metabolic Inflammation, Genetics, and Cardiometabolic Diseases. J Dent Res. 2015 Sep;94(9 Suppl):119S-27S.
85. Fentoğlu O, Sözen T, Oz SG, Kale B, Sönmez Y, Tonguç MO, et al. Short-term effects of periodontal therapy as an adjunct to anti-lipemic treatment. Oral Dis. 2010 Oct;16(7):648–54.
86. Park Y, Kim TJ, Lee H, Yoo H, Sohn I, Min YW, et al. Eradication of Helicobacter pylori infection decreases risk for dyslipidemia: A cohort study. Helicobacter. 2021 Apr;26(2):e12783.
87. Dancy C, Lohsoonthorn V, Williams MA. Risk of dyslipidemia in relation to level of physical activity among Thai professional and office workers. Southeast Asian J Trop Med Public Health. 2008 Sep;39(5):932–41.
88. Meireles De Pontes L. Standard of physical activity and influence of sedentarism in the occurrence of dyslipidemias in adults. Fit Perform J. 2008 Jul 1;7(4):245–50.
89. Zhou J, Zhou Q, Wang DP, Zhang T, Wang HJ, Song Y, et al. [Associations of sedentary behavior and physical activity with dyslipidemia]. Beijing Da Xue Xue Bao. 2017 Jun 18;49(3):418–23.
90. Wang X, Wang Y, Xu Z, Guo X, Mao H, Liu T, et al. Trajectories of 24-Hour Physical Activity Distribution and Relationship with Dyslipidemia. Nutrients. 2023 Jan 9;15(2):328.
91. Mutalifu M, Zhao Q, Wang Y, Hamulati X, Wang YS, Deng L, et al. Joint association of physical activity and diet quality with dyslipidemia: a cross-sectional study in Western China. Lipids Health Dis. 2024 Feb 10;23(1):46.
92. Churilla JR, Johnson TM, Zippel EA. Association of physical activity volume and hypercholesterolemia in US adults. QJM Mon J Assoc Physicians. 2013 Apr;106(4):333–40.
93. Gordon DJ, Witztum JL, Hunninghake D, Gates S, Glueck CJ. Habitual physical activity and high-density lipoprotein cholesterol in men with primary hypercholesterolemia. The Lipid Research Clinics Coronary Primary Prevention Trial. Circulation. 1983 Mar;67(3):512–20.
94. Delavar M, Lye M, Hassan S, Khor G, Hanachi P. Physical activity, nutrition, and dyslipidemia in middle-aged women. Iran J Public Health. 2011 Dec;40(4):89–98.
95. Brenta G, Fretes O. Dyslipidemias and hypothyroidism. Pediatr Endocrinol Rev PER. 2014 Jun;11(4):390–9.
96. Neves C, Alves M, Medina JL, Delgado JL. Thyroid diseases, dyslipidemia and cardiovascular pathology. Rev Port Cardiol Orgao Of Soc Port Cardiol Port J Cardiol Off J Port Soc Cardiol. 2008 Oct;27(10):1211–36.
97. Peppa M, Betsi G, Dimitriadis G. Lipid abnormalities and cardiometabolic risk in patients with overt and subclinical thyroid disease. J Lipids. 2011;2011:575840.
98. Jung KY, Ahn HY, Han SK, Park YJ, Cho BY, Moon MK. Association between thyroid function and lipid profiles, apolipoproteins, and high-density lipoprotein function. J Clin Lipidol. 2017;11(6):1347–53.
99. Duntas LH, Brenta G. A Renewed Focus on the Association Between Thyroid Hormones and Lipid Metabolism. Front Endocrinol. 2018;9:511.
100. Liberopoulos EN, Elisaf MS. Dyslipidemia in patients with thyroid disorders. Horm Athens Greece. 2002;1(4):218–23.
101.Asranna A, Taneja RS, Kulshreshta B. Dyslipidemia in subclinical hypothyroidism and the effect of thyroxine on lipid profile. Indian J Endocrinol Metab. 2012 Dec;16(Suppl 2):S347-349.
102. Arnaldi G, Scandali VM, Trementino L, Cardinaletti M, Appolloni G, Boscaro M. Pathophysiology of dyslipidemia in Cushing’s syndrome. Neuroendocrinology. 2010;92 Suppl 1:86–90.
103. Marcondes FK, Das Neves VJ, Costa R, Sanches A, Sousa T, Sampaio Moura MJC, et al. Dyslipidemia Induced by Stress. In: Kelishadi R, editor. InTech; 2012 [cited 2025 Jan 5]. Available from: http://www.intechopen.com/books/dyslipidemia-from-prevention-to-treatment/dyslipidemia-induced-by-stress
104. Nadolnik L, Polubok V, Gonchar K. Blood Cortisol Level in Patients with Metabolic Syndrome and Its Correlation with Parameters of Lipid and Carbohydrate Metabolisms. Int J Biochem Res Rev. 2020 Dec 31;149–58.
105. Veen G, Giltay EJ, DeRijk RH, van Vliet IM, van Pelt J, Zitman FG. Salivary cortisol, serum lipids, and adiposity in patients with depressive and anxiety disorders. Metabolism. 2009 Jun;58(6):821–7.
106. Christeff N, Melchior JC, de Truchis P, Perronne C, Nunez EA, Gougeon ML. Lipodystrophy defined by a clinical score in HIV-infected men on highly active antiretroviral therapy: correlation between dyslipidaemia and steroid hormone alterations. AIDS Lond Engl. 1999 Nov 12;13(16):2251–60.
107. Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. 2000 Apr;16(4):505–11.
108. Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009 Aug;94(8):2692–701.
109. van der Valk ES, Savas M, van Rossum EFC. Stress and Obesity: Are There More Susceptible Individuals? Curr Obes Rep. 2018 Jun;7(2):193–203.
110. Manenschijn L, Schaap L, van Schoor NM, van der Pas S, Peeters GMEE, Lips P, et al. High long-term cortisol levels, measured in scalp hair, are associated with a history of cardiovascular disease. J Clin Endocrinol Metab. 2013 May;98(5):2078–83.
111. Vicennati V, Pasqui F, Cavazza C, Pagotto U, Pasquali R. Stress-related development of obesity and cortisol in women. Obes Silver Spring Md. 2009 Sep;17(9):1678–83.
112. Torosyan N, Visrodia P, Torbati T, Minissian MB, Shufelt CL. Dyslipidemia in midlife women: Approach and considerations during the menopausal transition. Maturitas. 2022 Dec;166:14–20.
113. Meng Y, Lv PP, Ding GL, Yu TT, Liu Y, Shen Y, et al. High Maternal Serum Estradiol Levels Induce Dyslipidemia in Human Newborns via a Hepatic HMGCR Estrogen Response Element. Sci Rep. 2015 May 11;5:10086.
114. Schaefer EJ, Foster DM, Zech LA, Lindgren FT, Brewer HB, Levy RI. The effects of estrogen administration on plasma lipoprotein metabolism in premenopausal females. J Clin Endocrinol Metab. 1983 Aug;57(2):262–7.
115. Wahl P, Walden C, Knopp R, Hoover J, Wallace R, Heiss G, et al. Effect of estrogen/progestin potency on lipid/lipoprotein cholesterol. N Engl J Med. 1983 Apr 14;308(15):862–7.
116. Henriksson P, Stamberger M, Eriksson M, Rudling M, Diczfalusy U, Berglund L, et al. Oestrogen-induced changes in lipoprotein metabolism: role in prevention of atherosclerosis in the cholesterol-fed rabbit. Eur J Clin Invest. 1989 Aug;19(4):395–403.
117. Kushwaha RS, Hazzard WR. Exogenous estrogens attenuate dietary hypercholesterolemia and atherosclerosis in the rabbit. Metabolism. 1981 Apr;30(4):359–66.
118. Mudali S, Dobs AS, Ding J, Cauley JA, Szklo M, Golden SH, et al. Endogenous postmenopausal hormones and serum lipids: the atherosclerosis risk in communities study. J Clin Endocrinol Metab. 2005 Feb;90(2):1202–9.
119. Applebaum-Bowden D, McLean P, Steinmetz A, Fontana D, Matthys C, Warnick GR, et al. Lipoprotein, apolipoprotein, and lipolytic enzyme changes following estrogen administration in postmenopausal women. J Lipid Res. 1989 Dec;30(12):1895–906.
120. Gandarias JM, Abad C, Lacort M, Ochoa B. [Effect of progesterone on rat plasma and liver lipid levels (author’s transl)]. Rev Esp Fisiol. 1979 Dec;35(4):470–3.
121. Metherall JE, Waugh K, Li H. Progesterone inhibits cholesterol biosynthesis in cultured cells. Accumulation of cholesterol precursors. J Biol Chem. 1996 Feb 2;271(5):2627–33.
122. Abreu JM, Santos GB, Carvalho MDGDS, Mencarelli JM, Cândido BRM, Prado BBDP, et al. Dyslipidemia’s influence on the secretion ovarian’s steroids in female mice. Res Soc Dev. 2021 Oct 12;10(13):e298101321369.
123. Jensen JT, Addis IB, Hennebold JD, Bogan RL. Ovarian Lipid Metabolism Modulates Circulating Lipids in Premenopausal Women. J Clin Endocrinol Metab. 2017 Sep 1;102(9):3138–45.
124. Soma MR, Osnago-Gadda I, Paoletti R, Fumagalli R, Morrisett JD, Meschia M, et al. The lowering of lipoprotein[a] induced by estrogen plus progesterone replacement therapy in postmenopausal women. Arch Intern Med. 1993 Jun 28;153(12):1462–8.
125. Grönroos M, Lehtonen A. Effect of high dose progestin on serum lipids. Atherosclerosis. 1983 Apr;47(1):101–5.
126. Srinivasan SR, Sundaram GS, Williamson GD, Webber LS, Berenson GS. Serum lipoproteins and endogenous sex hormones in early life: observations in children with different lipoprotein profiles. Metabolism. 1985 Sep;34(9):861–7.
127. Thompson DL, Snead DB, Seip RL, Weltman JY, Rogol AD, Weltman A. Serum lipid levels and steroidal hormones in women runners with irregular menses. Can J Appl Physiol Rev Can Physiol Appl. 1997 Feb;22(1):66–77.
128. Haring R, Baumeister SE, Völzke H, Dörr M, Felix SB, Kroemer HK, et al. Prospective association of low total testosterone concentrations with an adverse lipid profile and increased incident dyslipidemia. Eur J Cardiovasc Prev Rehabil Off J Eur Soc Cardiol Work Groups Epidemiol Prev Card Rehabil Exerc Physiol. 2011 Feb;18(1):86–96.
129. Zhang N, Zhang H, Zhang X, Zhang B, Wang F, Wang C, et al. The relationship between endogenous testosterone and lipid profile in middle-aged and elderly Chinese men. Eur J Endocrinol. 2014 Apr;170(4):487–94.
130. Page ST, Mohr BA, Link CL, O’Donnell AB, Bremner WJ, McKinlay JB. Higher testosterone levels are associated with increased high-density lipoprotein cholesterol in men with cardiovascular disease: results from the Massachusetts Male Aging Study. Asian J Androl. 2008 Mar;10(2):193–200.
131. Nordøy A, Aakvaag A, Thelle D. Sex hormones and high density lipoproteins in healthy males. Atherosclerosis. 1979 Dec;34(4):431–6.
132. Cai Z, Xi H, Pan Y, Jiang X, Chen L, Cai Y, et al. Effect of testosterone deficiency on cholesterol metabolism in pigs fed a high-fat and high-cholesterol diet. Lipids Health Dis. 2015 Mar 7;14:18.
133. Self A, Zhang J, Corti M, Esani M. Correlation between Sex Hormones and Dyslipidemia. Am Soc Clin Lab Sci. 2019 Oct 14;ascls.119.002071.
134. Monroe AK, Dobs AS. The effect of androgens on lipids. Curr Opin Endocrinol Diabetes Obes. 2013 Apr;20(2):132–9.
135. Gutai J, LaPorte R, Kuller L, Dai W, Falvo-Gerard L, Caggiula A. Plasma testosterone, high density lipoprotein cholesterol and other lipoprotein fractions. Am J Cardiol. 1981 Nov;48(5):897–902.
Orthomolecular Medicine
Orthomolecular medicine uses safe, effective nutritional therapy to fight illness. For more information: http://www.orthomolecular.org
Find a Doctor
To locate an orthomolecular physician near you: http://orthomolecular.org/resources/omns/v06n09.shtml
The peer-reviewed Orthomolecular Medicine News Service is a non-profit and non-commercial informational resource.
Editorial Review Board:
Albert G. B. Amoa, MB.Ch.B, Ph.D. (Ghana)
Seth Ayettey, M.B., Ch.B., Ph.D. (Ghana)
Ilyès Baghli, M.D. (Algeria)
Barry Breger, M.D. (Canada)
Ian Brighthope, MBBS, FACNEM (Australia)
Gilbert Henri Crussol, D.M.D. (Spain)
Carolyn Dean, M.D., N.D. (USA)
Ian Dettman, Ph.D. (Australia)
Susan R. Downs, M.D., M.P.H. (USA)
Ron Ehrlich, B.D.S. (Australia)
Hugo Galindo, M.D. (Colombia)
Gary S. Goldman, Ph.D. (USA)
William B. Grant, Ph.D. (USA)
Claus Hancke, MD, FACAM (Denmark)
Patrick Holford, BSc (United Kingdom)
Ron Hunninghake, M.D. (USA)
Bo H. Jonsson, M.D., Ph.D. (Sweden)
Dwight Kalita, Ph.D. (USA)
Felix I. D. Konotey-Ahulu, M.D., FRCP (Ghana)
Peter H. Lauda, M.D. (Austria)
Fabrice Leu, N.D., (Switzerland)
Alan Lien, Ph.D. (Taiwan)
Homer Lim, M.D. (Philippines)
Stuart Lindsey, Pharm.D. (USA)
Pedro Gonzalez Lombana, M.D., Ph.D. (Colombia)
Victor A. Marcial-Vega, M.D. (Puerto Rico)
Juan Manuel Martinez, M.D. (Colombia)
Mignonne Mary, M.D. (USA)
Dr.Aarti Midha M.D., ABAARM (India)
Jorge R. Miranda-Massari, Pharm.D. (Puerto Rico)
Karin Munsterhjelm-Ahumada, M.D. (Finland)
Sarah Myhill, MB, BS (United Kingdom)
Tahar Naili, M.D. (Algeria)
Zhiyong Peng, M.D. (China)
Isabella Akyinbah Quakyi, Ph.D. (Ghana)
Selvam Rengasamy, MBBS, FRCOG (Malaysia)
Jeffrey A. Ruterbusch, D.O. (USA)
Gert E. Schuitemaker, Ph.D. (Netherlands)
Thomas N. Seyfried, Ph.D. (USA)
Han Ping Shi, M.D., Ph.D. (China)
T.E. Gabriel Stewart, M.B.B.CH. (Ireland)
Jagan Nathan Vamanan, M.D. (India)
Andrew W. Saul, Ph.D. (USA), Founding Editor
Richard Cheng, M.D., Ph.D. (USA), Editor-In-Chief
Associate Editor: Robert G. Smith, Ph.D. (USA)
Editor, Japanese Edition: Atsuo Yanagisawa, M.D., Ph.D. (Japan)
Editor, Chinese Edition: Richard Cheng, M.D., Ph.D. (USA)
Editor, Norwegian Edition: Dag Viljen Poleszynski, Ph.D. (Norway)
Editor, Arabic Edition: Moustafa Kamel, R.Ph, P.G.C.M (Egypt)
Editor, Korean Edition: Hyoungjoo Shin, M.D. (South Korea)
Editor, Spanish Edition: Sonia Rita Rial, PhD (Argentina)
Editor, German Edition: Bernhard Welker, M.D. (Germany)
Associate Editor, German Edition: Gerhard Dachtler, M.Eng. (Germany)
Assistant Editor: Michael Passwater (USA)
Contributing Editor: Thomas E. Levy, M.D., J.D. (USA)
Contributing Editor: Damien Downing, M.B.B.S., M.R.S.B. (United Kingdom)
Contributing Editor: W. Todd Penberthy, Ph.D. (USA)
Contributing Editor: Ken Walker, M.D. (Canada)
Contributing Editor: Michael J. Gonzalez, N.M.D., Ph.D. (Puerto Rico)
Technology Editor: Michael S. Stewart, B.Sc.C.S. (USA)
Associate Technology Editor: Robert C. Kennedy, M.S. (USA)
Legal Consultant: Jason M. Saul, JD (USA)
Comments and media contact: editor@orthomolecular.org OMNS welcomes but is unable to respond to individual reader emails. Reader comments become the property of OMNS and may or may not be used for publication.
Click here to see a web copy of this news release: https://orthomolecular.acemlna.com/p_v.php?l=1&c=351&m=346&s=a8c8fe7bea3fdaa4efae896c7612b3de
This news release was sent to brenton.satman@gmail.com. If you no longer wish to receive news releases, please reply to this message with “Unsubscribe” in the subject line or simply click on the following link: unsubscribe . To update your profile settings click here .
This article may be reprinted free of charge provided 1) that there is clear attribution to the Orthomolecular Medicine News Service, and 2) that both the OMNS free subscription link http://orthomolecular.org/subscribe.html and also the OMNS archive link http://orthomolecular.org/resources/omns/index.shtml are included.
Riordan Clinic | Orthomolecular.org
3100 N Hillside Ave
Wichita, Kansas 67219
United States
Reigniting Hope: Managing Systemic Lupus Erythematosus with Integrative Orthomolecular Medicine
Reproduced from original article:
https://orthomolecular.acemlna.com/p_v.php?l=1&c=333&m=337&s=a8c8fe7bea3fdaa4efae896c7612b3de
Orthomolecular Medicine News Service, November 16, 2024
Richard Z. Cheng, M.D., Ph.D.
OMNS (Nov 16, 2024) A recent story from Shanghai highlights the difficult journey of SLE patients. A woman named Shabai, after two decades battling this autoimmune disease and suffering kidney failure requiring dialysis, sought relief through assisted death in Switzerland. In her final social media post on October 24, 2024, she expressed gratitude for a ‘wonderful life,’ offering a heartfelt farewell with her father. Shabai’s story has ignited public empathy, underscoring the profound impact of SLE on physical and emotional well-being.
Having been asked to write about SLE, I aim to explore how integrative orthomolecular medicine can offer effective management strategies for this complex condition. Through a holistic approach that addresses root causes, nutrient support, and lifestyle factors, integrative orthomolecular medicine opens new avenues for reducing symptoms and enhancing quality of life. Patients with SLE should not give up hope; there are promising strategies that can empower them to live healthier, fuller lives despite their diagnosis.
Introduction: Systemic Lupus Erythematosus (SLE) is a complex autoimmune disease characterized by the production of autoantibodies and immune complex formation, affecting multiple organ systems.
While the exact etiology remains unclear, an integrative orthomolecular approach can provide insights into the root causes and intermediary mechanisms involved in SLE development and progression.
Root Causes Contributing to SLE:
- Unhealthy Diet: High consumption of carbohydrates, omega-6 polyunsaturated fatty acids (PUFAs), and ultra-processed foods may contribute to inflammation and immune dysregulation in SLE (1).
- A high-carbohydrate diet is associated with increased SLE risk (2). Specifically, women in the highest quintile of carbohydrate consumption had a nearly twofold increased risk compared to those in the lowest quintile (2). This suggests that diets high in carbohydrates may contribute to the development of SLE in this population.
- Overconsumption of omega-6 seed oil: Research suggests that an imbalanced omega-6 to omega-3 ratio, a global trend, may contribute to autoimmune diseases like systemic lupus erythematosus (SLE) (3). Omega-3 fatty acids have anti-inflammatory properties and may benefit autoimmune conditions (4-6). Research on mice demonstrated that omega-3-rich diets could reduce autoantibody production and kidney damage in SLE models (7). The complex relationship between dietary fats and autoimmunity is further highlighted by findings that both excessive omega-6 intake and reduced omega-3 consumption may exacerbate autoimmune diseases (8).
- Ultra-processed foods: Recent studies suggest a link between ultra-processed food (UPF) intake and increased risk of systemic lupus erythematosus (SLE), particularly in women. Higher UPF consumption was associated with a >50% increased SLE risk and doubled risk for anti-dsDNA+ SLE (9).
- Dietary toxins in plant-based foods: Lectins, found in many plant-based foods, have been identified as potential contributors to autoimmune diseases, including systemic lupus erythematosus (SLE). These carbohydrate-binding proteins can resist digestion, enter the bloodstream, and trigger immune responses (10,11). Lectins may disrupt intestinal barrier integrity, leading to various autoimmunities (11). While some researchers caution against labeling plant compounds as “anti-nutrients” (12), others emphasize the potential risks of lectins, oxalates, and other plant-based toxins (13,14). Natural plant metabolites have been explored as potential remedies for SLE due to their immunomodulatory properties (15). Environmental factors, including toxic chemicals, are believed to contribute significantly to autoimmune diseases (16). Oxidative stress, arising from both endogenous and exogenous sources, has been identified as a unifying theme in the pathogenesis of SLE and other autoimmune conditions (17).
- Environmental Toxins: Exposure to chemicals, pesticides, and heavy metals may trigger autoimmune responses. Occupational exposure to crystalline silica has been studied as a possible trigger for SLE (18).
- Environmental toxins and chemicals have been implicated in the development and exacerbation of systemic lupus erythematosus (SLE) and other autoimmune conditions. Various studies have linked exposure to silica, solvents, pesticides, heavy metals, and endocrine disruptors like bisphenol A (BPA) and bisphenol F (BPF) to increased SLE risk (19-21). These toxins can trigger autoimmunity through multiple mechanisms, including epigenetic alterations, immune dysregulation, antioxidant depletion, and barrier degradation in genetically susceptible individuals (22). Cigarette smoking, oral contraceptives, and postmenopausal hormone therapy have also been associated with SLE incidence, while alcohol consumption may decrease risk (23). Environmental exposures can lead to chronic inflammation, tissue damage, and the release of self-antigens, potentially contributing to the development of autoimmunity (24). Further research is needed to fully elucidate the complex interactions between environmental factors and genetic susceptibility in SLE pathogenesis (25).
- Heavy metals exposure has been linked to autoimmune diseases, including SLE (20,26). Metals such as mercury, cadmium, and lead can disrupt immune responses, potentially exacerbating immune tolerance issues and chronic inflammation (27-29). These metals can affect both innate and adaptive immunity, leading to chronic inflammation and disrupted immune tolerance (30,31). This exposure triggers immune dysregulation through pathways like oxidative stress, genetic predisposition, and epigenetic alterations (26,31,32).
- Infections: Infections play a crucial role in the etiopathogenesis and exacerbation of systemic lupus erythematosus (SLE) (33,34). Various pathogens, particularly viruses like Epstein-Barr virus, can trigger autoimmunity through molecular mimicry and immune dysregulation (35,36). SLE patients are more susceptible to infections due to genetic factors and immunosuppressive treatments (37). Bacterial infections, including periodontal disease, may contribute to SLE pathogenesis by exposing nuclear autoantigens and stimulating Toll-like receptors (TLRs) 2 and 4 (38,39). Periodontal disease is associated with increased inflammatory markers and may be a risk factor for cardiovascular disease in SLE patients (40). Preventive measures, such as screening for chronic infections before immunosuppressive therapy, are crucial in managing SLE patients (35,37).
- Nutrient Deficiencies: Insufficient intake of vitamins and micronutrients, especially B vitamins, vitamins C, D3, and K2, as well as magnesium and selenium, may contribute to immune dysfunction in SLE (1). Vitamin D deficiency, in particular, has been linked to increased SLE activity (1). Vitamin D deficiency is prevalent in systemic lupus erythematosus (SLE) patients and associated with increased disease activity (41). Low vitamin D levels correlate with higher autoantibody production, B cell hyperactivity, and interferon-α activity in SLE patients (42). Vitamin D plays a crucial role in immune regulation and may contribute to autoimmune disease pathogenesis (43). Supplementation with vitamin D has shown potential in reducing inflammatory markers and disease activity in SLE patients (44). Factors such as photosensitivity, photoprotection, and postmenopausal status are associated with vitamin D deficiency in SLE patients (41,45). Hydroxychloroquine use may help prevent vitamin D deficiency (45). While the relationship between vitamin D and SLE is complex, addressing vitamin D deficiency may have benefits beyond bone health for SLE patients (46,47).
- Mental Health and Stress: Emotional or physical stress can trigger SLE flares (18). Chronic stress may contribute to immune system dysregulation.
- Genetics: SLE has a strong genetic component, with multiple genetic variants associated with increased susceptibility (18).
- Hormonal Imbalance: SLE often manifests or worsens during periods of hormonal fluctuations, such as puberty, pregnancy, or menopause (18). Hormonal imbalances play a significant role in the pathogenesis of autoimmune diseases, particularly systemic lupus erythematosus (SLE). The higher prevalence of SLE in women, especially during reproductive years, suggests a strong influence of sex hormones (48,49). Studies have shown that SLE patients exhibit abnormal hormone levels, including elevated estrogen and prolactin, and decreased androgens (50,51). These hormonal alterations affect both innate and adaptive immune responses, contributing to disease development and progression (52). Estrogen, in particular, can exert pro-inflammatory effects through genomic and non-genomic pathways, influencing B cell maturation and selection (53,54). Additionally, environmental factors such as estrogenic endocrine disruptors may trigger or alter autoimmune disease onset (53). The complex interplay between sex hormones, cytokines, and the immune system highlights the importance of hormonal balance in SLE pathogenesis (55). Notably, dehydroepiandrosterone (DHEA) has shown promise as a potential treatment for SLE. Multiple studies have demonstrated that DHEA supplementation (200 mg/day) can reduce disease activity, decrease corticosteroid requirements, and improve health-related quality of life in SLE patients (56-59). DHEA has also been found to have a protective effect against corticosteroid-induced osteopenia (58).
- Ultraviolet Radiation: Sunlight, particularly UVB rays, is a well-established trigger for SLE flares (18).
- Lifestyle Factors: Smoking is both a potential flare trigger and a risk factor for SLE, increasing the risk of skin and kidney problems (18).
Intermediary Mechanisms in SLE
- Leaky Gut: Increased intestinal permeability, or “leaky gut,” is a common underlying cause of autoimmunity (60). In SLE, this can lead to undigested food particles entering the bloodstream, triggering immune responses and potentially causing molecular mimicry.
- Elevated Oxidative Stress: SLE patients exhibit high levels of oxidative stress, which can damage cellular components and contribute to inflammation (61). This may be exacerbated by nutrient deficiencies and environmental toxins.
- Impaired Mitochondrial Function: Mitochondrial dysfunction has been implicated in various autoimmune diseases and may play a role in SLE pathogenesis (62).
- Insulin Resistance in SLE: Insulin resistance (IR) is more prevalent in systemic lupus erythematosus (SLE) patients compared to healthy controls, increasing the risk of cardiovascular disease and type 2 diabetes mellitus (63). SLE patients exhibit higher C-peptide levels and elevated HOMA2-IR-C-peptide index, independent of traditional cardiovascular risk factors (64). IR in SLE is associated with disease activity, inflammation markers, and damage over time (64,65). Oxidative stress, indicated by increased malondialdehyde levels, correlates with IR in SLE patients (66). Type B insulin resistance syndrome, characterized by autoantibodies to insulin receptors, can occur in SLE patients and may respond to immunosuppressive treatment (67,68). Cyclophosphamide and mycophenolate mofetil have been successfully used to treat SLE-associated type B insulin resistance (69). Understanding these mechanisms can lead to better treatment strategies for SLE patients with IR.
- Immune System Dysregulation: SLE is characterized by an imbalance of T-helper cell subsets (Th1/Th2/Th17) and regulatory T-cells (Tregs), contributing to tissue damage and increased proinflammatory responses (1).
- Autoantibody Production: The hallmark of SLE is the production of autoantibodies, particularly antinuclear antibodies (ANA), which target the body’s own tissues (18).
- Complement Activation: Intravascular activation and conversion of complement contribute to increased capillary permeability and tissue damage in SLE (61).
- Cytokine Imbalance: SLE patients exhibit elevated levels of proinflammatory cytokines, including IFN-γ, TNF, IL-4, IL-6, IL-10, IL-12, IL-17, and IL-18, while IL-2 levels are typically lower compared to healthy controls (1).
Integrative Intervention:
- Healthy diet: A 2022 study found that low carbohydrate intake, improved self-reported symptoms in SLE patients (70). While not specific to SLE, a 2023 case report on a very low calorie ketogenic diet (VLCKD) for rheumatic disorders found: “VLCKD allowed the patient to achieve weight goal, better management of joint pain, headache episodes and normalization of inflammatory indices (71). A review on diet and SLE management stated: “Currently, a diet rich in vitamin- and mineral-rich foods and MUFA/PUFA with moderate energy consumption is recommended to control the inflammatory findings of the disease and the complications and co-morbidities resulting from SLE therapy” (1).
- Nutritional supplements: Supplementation of vitamins, micronutrients, antioxidants and mitochondrial nutrients, often at high doses, has shown various effectiveness on autoimmune diseases, including SLE.
- High dose vitamin B1 (thiamine): High-dose thiamine has shown benefits for autoimmune diseases like rheumatoid arthritis, lupus, and Hashimoto’s thyroiditis. These findings suggest potential broader applications for autoimmune skin conditions (72-79).
- High dose vitamin B2 (riboflavin): High-dose vitamin B2 (riboflavin) has shown potential benefits in managing autoimmune diseases, primarily due to its role in reducing oxidative stress, supporting mitochondrial function, and modulating immune responses (80-82).
- High dose vitamin B3 (niacin/nicotinamide): Vitamin B3 (niacin/nicotinamide) shows promise in treating various autoimmune and inflammatory conditions. High doses of nicotinamide can reduce regulatory T cells and alter immune tolerance (83). In dermatology, it has been used to treat autoimmune skin diseases in dogs (84) and shows potential for treating acne, rosacea, and photoaging in humans (85,86). Nicotinamide has also been investigated for preventing type 1 diabetes (87) and as a cytoprotectant in immune system disorders (88). Recent studies demonstrate its ability to suppress T cell activation and pro-inflammatory cytokine production in juvenile idiopathic arthritis (89). Additionally, niacin has shown potential in enhancing remyelination in aging central nervous systems by rejuvenating macrophage/microglia function (90). These findings suggest that vitamin B3 may have therapeutic applications across various autoimmune and inflammatory conditions.
- High dose vitamin B5 (pantothenic acid): Recent research suggests a potential role for vitamin B5 and vitamin D in autoimmune diseases, including systemic lupus erythematosus (SLE). Vitamin B5 has been shown to inhibit Th17 cell differentiation and related autoimmune diseases by impeding PKM2 nuclear translocation (91). It may also have paradoxical effects on inflammatory and anti-inflammatory cytokines (29). Vitamin B5 deficiency can have significant health consequences (92).
- High dose vitamin B6 (pyridoxine): Research suggests that vitamin B6 supplementation may have beneficial effects for autoimmune conditions like systemic lupus erythematosus (SLE). Higher intake of vitamin B6 was associated with a reduced risk of active disease in SLE patients (93). High-dose vitamin B6 demonstrated strong anti-inflammatory properties in monocytes by downregulating key inflammatory mediators (94). It also prevented excessive inflammation by reducing sphingosine-1-phosphate accumulation (95). In critically ill patients, vitamin B6 supplementation increased immune responses (96). Vitamin B6 deficiency is associated with inflammation, and supplementation may improve immune function (97). However, very high doses of vitamin B6 can cause peripheral neuropathy, so appropriate dosing is crucial (98).
- High dose vitamin B7 (biotin): Recent research suggests potential benefits of vitamin B7 supplementation for autoimmune disorders. High-dose biotin (vitamin B7) has shown promise in treating progressive multiple sclerosis by promoting remyelination and enhancing energy production (99), although studies of biotin on SLE are limited.
- High dose vitamin C (ascorbic acid): Recent studies suggest that vitamin C supplementation may have beneficial effects in treating autoimmune diseases like Systemic Lupus Erythematosus (SLE) and rheumatoid arthritis by regulating cytokines, modulating immune responses, and reducing oxidative stress (100). High-dose vitamin C treatment has been shown to increase glucocorticoid activity and potentially control autoimmune diseases (101). In SLE patients, combined vitamin C and E supplementation decreased lipid peroxidation but did not affect endothelial function (102). Vitamin C intake was inversely associated with SLE disease activity in a 4-year prospective study (103).
- High dose vitamin D: Recent research suggests a potential role for vitamin D in autoimmune diseases, including systemic lupus erythematosus (SLE). Vitamin D deficiency has been associated with autoimmune disorders, including SLE (104). Up to 69% of SLE patients were found to be vitamin D deficient in one study, compared to only 22% of healthy controls without antinuclear antibodies (ANA) (42). While a randomized trial found no significant effect of high-dose vitamin D on SLE disease activity, it did demonstrate a corticosteroid-sparing effect (105). Some SLE patients develop anti-vitamin D antibodies, which are associated with anti-dsDNA antibodies (106). The concept of acquired vitamin D resistance may explain the need for high-dose vitamin D therapy in autoimmune diseases (107,108). Vitamin D supplementation is increasingly recommended for SLE patients (109).
- The recent trials of CAM treatments for SLE indicate that supplements such as vitamin D, omega 3 fatty acids, N-acetyl cysteine and turmeric show some promise for reducing SLE disease activity (80).
- PBMT (Photobiomodulation therapy): PBMT shows promise in treating autoimmune diseases like multiple sclerosis (MS) and systemic lupus erythematosus (SLE). Studies demonstrate that PBMT, particularly using wavelengths of 670nm and 830nm, can modulate immune responses by increasing anti-inflammatory cytokines like IL-10 and decreasing pro-inflammatory cytokines such as IFN-γ (110,111). PBMT also reduces nitric oxide production, potentially alleviating nitrosative stress in MS patients (111). In experimental autoimmune encephalomyelitis, a mouse model of MS, 670nm light treatment reduced disease severity and modulated cytokine production (112). For SLE, both extracorporeal photochemotherapy and ultraviolet-A1 irradiation therapy have shown clinical improvements (113). Additionally, photodynamic therapy with 5-aminolevulinic acid successfully treated skin ulcers in an SLE patient (114). These findings suggest that various forms of light therapy could be valuable in managing autoimmune diseases.
- Methyelene Blue: Recent research suggests that methylene blue and metabolic modulators may have therapeutic potential for systemic lupus erythematosus (SLE) and other autoimmune diseases. Methylene blue has shown promise in reducing symptoms of experimental autoimmune encephalomyelitis by modulating immune responses and activating the AMPK/SIRT1 pathway (115). Metabolic disturbances, including oxidative stress and altered lipid profiles, have been observed in SLE patients (53). Normalizing T cell metabolism through inhibition of glycolysis and mitochondrial metabolism has demonstrated efficacy in treating lupus in animal models and human cells (116). Other potential therapeutic approaches include methimazole, which prevents experimental SLE in mice (117), and histone deacetylase inhibitors, which may reverse epigenetic dysregulation in SLE (118). DNA methylation patterns have also emerged as important biomarkers and potential therapeutic targets in SLE (119).
- Stem cell therapy for SLE: Stem cell therapy, particularly using mesenchymal stem cells (MSCs), has shown promise in treating systemic lupus erythematosus (SLE) (120), a chronic autoimmune disease affecting multiple organs. MSCs demonstrate immunomodulatory effects, inhibiting inflammatory factors and pathways while promoting regulatory T cells (121-123). Clinical trials have indicated that MSC therapy is generally safe and can improve disease activity, reduce autoantibodies, and ameliorate organ dysfunction in SLE patients (123,124). However, challenges remain, including potential complications and variable efficacy (123,124). Further research is needed to optimize stem cell therapy for SLE, including investigating MSC modification methods to enhance their immunosuppressive effects (121,125).
Summary of Key Benefits:
- Healthy Diets Low in Carbohydrates, Omega-6 PUFAs, Plant-Based Toxins, and Ultra-Processed Foods: A diet focused on low carbohydrates, reduced omega-6 polyunsaturated fatty acids (PUFAs), minimal plant-based toxins (like lectins and oxalates), and limited ultra-processed foods can help lower inflammation, support metabolic health, and improve immune regulation. This dietary approach may alleviate symptoms, reduce flare-ups, and promote overall well-being for individuals with SLE and other autoimmune conditions by addressing key dietary triggers of inflammation and immune dysregulation.
- Vitamin B1: Potential for reducing autoimmune symptoms.
- Vitamin B2: Supports oxidative stress reduction and immune modulation.
- Vitamin B3: Shows promise for treating inflammatory conditions.
- Vitamin B5: May inhibit inflammatory pathways.
- Vitamin B6: Anti-inflammatory effects with improved immune function.
- Vitamin B7: Promotes energy and remyelination in certain cases.
- Vitamin C: Reduces oxidative stress and supports immune modulation.
- Vitamin D: Associated with reduced disease activity and immune regulation benefits.
- PBMT (Photobiomodulation Therapy): Modulates immune response by increasing anti-inflammatory cytokines (like IL-10) and reducing pro-inflammatory cytokines. PBMT also supports cellular energy production and reduces oxidative stress, making it beneficial for managing inflammation and symptoms in SLE and other autoimmune conditions.
- Methylene Blue: Enhances mitochondrial function, reduces oxidative stress, and modulates immune responses. Methylene blue’s impact on the AMPK/SIRT1 pathway may support energy production and reduce inflammation, which could benefit autoimmune diseases, including SLE.
- Hormonal Balance: Hormonal balance helps regulate immune responses, reduces autoimmune disease activity, decreases corticosteroid needs, and improves quality of life, especially in conditions like SLE.
- Detox of Heavy Metals: Reduces the toxic load that may exacerbate autoimmune conditions. By eliminating metals like mercury, cadmium, and lead, patients can improve immune tolerance, decrease chronic inflammation, and support overall immune system health.
Conclusion: Addressing root causes and intermediary mechanisms in SLE through integrative methods offers promise for improved outcomes. By combining nutritional, environmental, and lifestyle modifications with targeted interventions for immune regulation and oxidative stress, SLE patients may experience relief and enhanced quality of life. Integrative orthomolecular medicine presents a holistic, patient-centered approach to nurturing resilience and optimism in the face of chronic autoimmune challenges.
Through this integrative orthomolecular approach, we have observed significant improvements in our patients’ quality of life (126,127). In many cases, these methods have even contributed to reversing symptoms of various autoimmune diseases. This experience reinforces the potential of integrative medicine to provide renewed hope and health to those facing the challenges of autoimmune conditions.
References:
1. Aparicio-Soto M, Sánchez-Hidalgo M, Alarcón-de-la-Lastra C. An update on diet and nutritional factors in systemic lupus erythematosus management. Nutr Res Rev. 2017 Jun;30(1):118-37.
2. Castro-Webb N, Cozier YC, Barbhaiya M, Ruiz-Narváez EA, Li S, Costenbader KH, et al. Association of macronutrients and dietary patterns with risk of systemic lupus erythematosus in the Black Women’s Health Study. Am J Clin Nutr. 2021 Oct 1;114(4):1486-94.
3. DiNicolantonio JJ, O’Keefe J. The Importance of Maintaining a Low Omega-6/Omega-3 Ratio for Reducing the Risk of Autoimmune Diseases, Asthma, and Allergies. Mo Med. 2021;118(5):453-9.
4. Simopoulos AP. Omega-3 Fatty Acids in Inflammation and Autoimmune Diseases. J Am Coll Nutr. 2002 Dec 1;21(6):495-505.
5. Pestka JJ, Vines LL, Bates MA, He K, Langohr I. Comparative effects of n-3, n-6 and n-9 unsaturated fatty acid-rich diet consumption on lupus nephritis, autoantibody production and CD4+ T cell-related gene responses in the autoimmune NZBWF1 mouse. PloS One. 2014;9(6):e100255.
6. Liu A, Li Z, Zeng J, Peng Y, Wang S, Bi X, et al. ω-3 polyunsaturated fatty acid alleviates systemic lupus erythematosus by suppressing autoimmunity in a murine model. Int Immunopharmacol. 2024 Jan 5;126:111299.
7. Reifen R, Blank M, Afek A, Kopilowiz Y, Sklan D, Gershwin ME, et al. Dietary polyunsaturated fatty acids decrease anti-dsDNA and anti-cardiolipin antibodies production in idiotype induced mouse model of systemic lupus erythematosus. Lupus. 1998;7(3):192-7.
8. Fernandes G. Dietary lipids and risk of autoimmune disease. Clin Immunol Immunopathol. 1994 Aug;72(2):193-7.
9. Rossato S, Oakes EG, Barbhaiya M, Sparks JA, Malspeis S, Willett WC, et al. Ultraprocessed Food Intake and Risk of Systemic Lupus Erythematosus Among Women Observed in the Nurses’ Health Study Cohorts. Arthritis Care Res. 2024 Jun 27;
10. Hamid R, Masood A. Dietary Lectins as Disease Causing Toxicants [Internet]. [cited 2024 Nov 3]. Available from: https://scialert.net/abstract/?doi=pjn.2009.293.303
11. Vojdani A. Lectins, agglutinins, and their roles in autoimmune reactivities. Altern Ther Health Med. 2015;21 Suppl 1:46-51.
12. Petroski W, Minich DM. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients. 2020 Oct;12(10):2929.
13. Popova A, Mihaylova D. Antinutrients in Plant-based Foods: A Review. [cited 2024 Nov 3]; Available from: https://openbiotechnologyjournal.com/VOLUME/13/PAGE/68/
14. Freed DLJ. Lectins in Food: Their Importance in Health and Disease. J Nutr Med. 1991 Jan 1;2(1):45-64.
15. Balkrishna A, Thakur P, Singh S, Chandra Dev SN, Varshney A. Mechanistic Paradigms of Natural Plant Metabolites as Remedial Candidates for Systemic Lupus Erythromatosus. Cells. 2020 Apr;9(4):1049.
16. Petric D. Review of Toxins Associated with Autoimmune Diseases. Sci Prepr [Internet]. 2021 Oct 21 [cited 2024 Nov 3]; Available from: https://www.scienceopen.com/hosted-document?doi=10.14293/S2199-1006.1.SOR-.PPMAW3U.v1
17. Kovacic P, Jacintho JD. Systemic lupus erythematosus and other autoimmune diseases from endogenous and exogenous agents: unifying theme of oxidative stress. Mini Rev Med Chem. 2003 Sep;3(6):568-75.
18. Mount S. Mount Sinai Health System. [cited 2024 Nov 3]. Systemic lupus erythematosus Information | Mount Sinai – New York. Available from: https://www.mountsinai.org/health-library/report/systemic-lupus-erythematosus
19. Mak A, Tay SH. Environmental Factors, Toxicants and Systemic Lupus Erythematosus. Int J Mol Sci. 2014 Sep;15(9):16043-56.
20. Pan Q, Guo Y, Guo L, Liao S, Zhao C, Wang S, et al. Mechanistic Insights of Chemicals and Drugs as Risk Factors for Systemic Lupus Erythematosus. Curr Med Chem. 27(31):5175-88.
21. Wang Y, Wu H, Li K, Huang R, Liu J, Lu Z, et al. Environmental triggers of autoimmunity: The association between bisphenol analogues and systemic lupus erythematosus. Ecotoxicol Environ Saf. 2024 Jun 15;278:116452.
22. Kharrazian D. Exposure to Environmental Toxins and Autoimmune Conditions. Integr Med Encinitas Calif. 2021 Apr;20(2):20-4.
23. Barbhaiya M, Costenbader KH. Environmental exposures and the development of systemic lupus erythematosus. Curr Opin Rheumatol. 2016 Sep;28(5):497.
24. Pollard KM, Christy JM, Cauvi DM, Kono DH. Environmental xenobiotic exposure and autoimmunity. Curr Opin Toxicol. 2018 Aug 1;10:15-22.
25. Sarzi-Puttini P, Iaccarino L. Environment and systemic lupus erythematosus: An overview: Autoimmunity: Vol 38 , No 7 – Get Access [Internet]. [cited 2024 Nov 3]. Available from: https://www.tandfonline.com/doi/full/10.1080/08916930500285394
26. Liu JL, Woo JMP, Parks CG, Costenbader KH, Jacobsen S, Bernatsky S. Systemic Lupus Erythematosus Risk: The Role of Environmental Factors. Rheum Dis Clin N Am. 2022 Nov 1;48(4):827-43.
27. Mishra KP, Singh SB. Heavy Metals Exposure and Risk of Autoimmune Diseases: A Review. Arch Immunol Allergy. 2020 Dec 3;3(2):22-6.
28. Bigazzi PE. Autoimmunity and heavy metals. Lupus. 1994 Dec;3(6):449-53.
29. Mishra KP. Lead exposure and its impact on immune system: A review. Toxicol In Vitro. 2009 Sep 1;23(6):969-72.
30. Anka AU, Usman AB. Potential mechanisms of some selected heavy metals in the induction of inflammation and autoimmunity – Abubakar U Anka, Abubakar B Usman, Abubakar N Kaoje, Ramadan M Kabir, Aliyu Bala, Mandana Kazem Arki, Nikoo Hossein-Khannazer, Gholamreza Azizi, 2022 [Internet]. [cited 2024 Nov 3]. Available from: https://journals.sagepub.com/doi/10.1177/1721727X221122719
31. Hemdan NYA, Emmrich F, Faber S, Lehmann J, Sack U. Alterations of Th1/Th2 Reactivity by Heavy Metals. Ann N Y Acad Sci. 2007;1109(1):129-37.
32. Cojocaru M, Chicoş B. The role of heavy metals in autoimmunity. Romanian J Intern Med Rev Roum Med Interne. 2014;52(3):189-91.
33. Caza T, Oaks Z, Perl A. Interplay of Infections, Autoimmunity, and Immunosuppression in Systemic Lupus Erythematosus: International Reviews of Immunology: Vol 33 , No 4 – Get Access. Rev Immunol. 2014 Jan 28;33(4):330-63.
34. Zandman-Goddard G, Shoenfeld Y, Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005 Jan 1;38(7):473-85.
35. Doria A, Canova M, Tonon M, Zen M, Rampudda E, Bassi N, et al. Infections as triggers and complications of systemic lupus erythematosus. Autoimmun Rev. 2008 Oct 1;8(1):24-8.
36. Rigante D, Esposito S. Infections and Systemic Lupus Erythematosus: Binding or Sparring Partners? Int J Mol Sci. 2015 Aug;16(8):17331-43.
37. Fessler BJ. Infectious diseases in systemic lupus erythematosus: risk factors, management and prophylaxis. Best Pract Res Clin Rheumatol. 2002 Apr 1;16(2):281-91.
38. Qiu C, Caricchio R, Gallucci S. Frontiers | Triggers of Autoimmunity: The Role of Bacterial Infections in the Extracellular Exposure of Lupus Nuclear Autoantigens. Front Immunol [Internet]. 2019 Nov 8 [cited 2024 Nov 3];10. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2019.02608/full
39. Marques CPC, Maor Y, de Andrade MS, Rodrigues VP, Benatti BB. Possible evidence of systemic lupus erythematosus and periodontal disease association mediated by Toll-like receptors 2 and 4. Clin Exp Immunol. 2016 Feb 1;183(2):187-92.
40. Pessoa L, Galvão V, Santos-Neto L. Periodontal disease as a risk factor for cardiovascular disease: Suggestion of a further link in systemic lupus erythematosus. Med Hypotheses. 2011 Aug 1;77(2):286-9.
41. Szodoray P, Tarr T, Bazso A, Poor G, Szegedi G, Kiss E. The immunopathological role of vitamin D in patients with SLE: data from a single centre registry in Hungary. Scand J Rheumatol. 2011 Mar;40(2):122-6.
42. Ritterhouse LL, Crowe SR, Niewold TB, Kamen DL, Macwana SR, Roberts VC, et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann Rheum Dis. 2011 Sep 1;70(9):1569-74.
43. Cutolo M, Otsa K. Review: Vitamin D, immunity and lupus [Internet]. 2008 [cited 2024 Nov 3]. Available from: https://journals.sagepub.com/doi/10.1177/0961203307085879
44. Abou-Raya S, Helmii M. The Effect of Vitamin D Supplementation on Inflammatory and Hemostatic Markers and Disease Activity in Patients with Systemic Lupus Erythematosus: A Randomized Placebo-controlled Trial. J Rheumatol. 2018 Dec;45(12):1713.
45. Ruiz-Irastorza G, Egurbide MV, Olivares N, Martinez-Berriotxoa A, Aguirre C. Vitamin D deficiency in systemic lupus erythematosus: prevalence, predictors and clinical consequences. Rheumatol Oxf Engl. 2008 Jun;47(6):920-3.
46. Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol. 2008 Sep;20(5):532-7.
47. Dall’Ara F, Cutolo M, Andreoli L, Tincani A, Paolino S. Vitamin D and systemic lupus erythematous: a review of immunological and clinical aspects. Clin Exp Rheumatol. 2018;36(1):153-62.
48. Katsuyama T, Moulton VR. Chapter 13 – Hormones. In: Tsokos GC, editor. Systemic Lupus Erythematosus (Second Edition) [Internet]. Academic Press; 2021 [cited 2024 Nov 3]. p. 105-12. Available from: https://www.sciencedirect.com/science/article/pii/B9780128145517000131
49. Moulton VR, Tsokos GC. Why do women get lupus? Clin Immunol. 2012 Jul 1;144(1):53-6.
50. Li J, May W, McMurray RW. Pituitary hormones and systemic lupus erythematosus. Arthritis Rheum. 2005 Dec;52(12):3701-12.
51. McMurray RW. Sex hormones in the pathogenesis of systemic lupus erythematosus. Front Biosci-Landmark. 2001 Dec 1;6(4):193-206.
52. Crispín JC, Liossis SNC, Kis-Toth K, Lieberman LA, Kyttaris VC, Juang YT, et al. Pathogenesis of human systemic lupus erythematosus: recent advances. Trends Mol Med. 2010 Feb 1;16(2):47-57.
53. Pierdominici M, Ortona E. Estrogen Impact on Autoimmunity Onset and Progression: the Paradigm of Systemic Lupus Erythematosus. In 2013 [cited 2024 Nov 3]. Available from: https://www.semanticscholar.org/paper/Estrogen-Impact-on-Autoimmunity-Onset-and-the-of-Pierdominici-Ortona/e7b114667e74573acb0db515547e993549971f50
54. Cohen-Solal JFG, Jeganathan V, Grimaldi CM, Peeva E, Diamond B. Sex Hormones and SLE: Influencing the Fate of Autoreactive B Cells. In: Radbruch A, Lipsky PE, editors. Current Concepts in Autoimmunity and Chronic Inflammation [Internet]. Berlin, Heidelberg: Springer; 2006 [cited 2024 Nov 3]. p. 67-88. Available from: https://doi.org/10.1007/3-540-29714-6_4
55. Cutolo M, Sulli A, Villaggio B, Seriolo B, Accardo S. Relations between steroid hormones and cytokines in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 1998 Oct 1;57(10):573-7.
56. van Vollenhoven RF, Engleman EG, McGuire JL. An open study of dehydroepiandrosterone in systemic lupus erythematosus. Arthritis Rheum. 1994 Sep;37(9):1305-10.
57. Van Vollenhoven RF, Engleman EG, Mcguire JL. Dehydroepiandrosterone in systemic lupus erythematosus. Arthritis Rheum. 1995;38(12):1826-31.
58. van Vollenhoven RF, Park JL, Genovese MC, West JP, McGuire JL. A double-blind, placebo-controlled, clinical trial of dehydroepiandrosterone in severe systemic lupus erythematosus. Lupus. 1999;8(3):181-7.
59. Crosbie D, Black C, McIntyre L, Royle PL, Thomas S. Dehydroepiandrosterone for systemic lupus erythematosus. Cochrane Database Syst Rev. 2007 Oct 17;2007(4):CD005114.
60. Caplan T, Caplan B. What Are the Main Triggers and Root Causes of Lupus? [Internet]. 2019. Available from: https://caplanhealthinstitute.com/leaky-gut-main-root-causes-lupus/
61. Tian XP, Zhang X. Gastrointestinal involvement in systemic lupus erythematosus: Insight into pathogenesis, diagnosis and treatment. World J Gastroenterol WJG. 2010 Jun 28;16(24):2971.
62. Halfon M, Tankeu AT, Ribi C. Mitochondrial Dysfunction in Systemic Lupus Erythematosus with a Focus on Lupus Nephritis. Int J Mol Sci. 2024 Jun 3;25(11):6162.
63. García-Carrasco M, Mendoza-Pinto C, Munguía-Realpozo P, Etchegaray-Morales I, Vélez-Pelcastre SK, Méndez-Martínez S, et al. Insulin Resistance and Diabetes Mellitus in Patients with Systemic Lupus Erythematosus. Endocr Metab Immune Disord – Drug Targets. 23(4):503-14.
64. Sánchez-Pérez H, Tejera-Segura B, de Vera-González A, González-Delgado A, Olmos JM, Hernández JL, et al. Insulin resistance in systemic lupus erythematosus patients: contributing factors and relationship with subclinical atherosclerosis. Clin Exp Rheumatol. 2017;35(6):885-92.
65. Dawood A, Fayez D, Essa E, El-zorkany K, El-Najjar M, Gazareen S. Study of insulin resistance in patients with systemic lupus erythematosus and rheumatoid arthritis. Menoufia Med J. 2014 Jun 1;27(2):215-25.
66. Koca SS, Karaca I, Yavuzkir MF, Dağli N, Ozgen M, Ustündağ B, et al. Insulin resistance is related with oxidative stress in systemic lupus erythematosus. Anadolu Kardiyol Derg AKD Anatol J Cardiol. 2009 Feb;9(1):23-8.
67. Kawashiri SY, Kawakami A, Fujikawa K, Iwamoto N, Aramaki T, Tamai M, et al. Type B insulin resistance complicated with systemic lupus erythematosus. Intern Med Tokyo Jpn. 2010;49(5):487-90.
68. Alvarez-Payares JC, Ribero D, Rodríguez L, Builes CE, Prieto C, Arango C, et al. Late Systemic Lupus Erythematosus-Associated Insulin Resistance Syndrome: A Rare Cause of De Novo Diabetes Mellitus. Case Rep Med. 2022;2022:4655804.
69. Gehi A, Webb A. Treatment of systemic lupus erythematosus-associated type B insulin resistance syndrome with cyclophosphamide and mycophenolate mofetil. Arthritis Rheum. 2003 Apr;48(4):1067-70.
70. Knippenberg A, Robinson GA, Wincup C, Ciurtin C, Jury EC, Kalea AZ. Plant-based dietary changes may improve symptoms in patients with systemic lupus erythematosus. Lupus. 2022 Jan 3;31(1):65.
71. Rondanelli M, Patelli Z, Gasparri C, Mansueto F, Ferraris C, Nichetti M, et al. Very low calorie ketogenic diet and common rheumatic disorders: A case report. World J Clin Cases. 2023 Mar 26;11(9):1985.
72. Queen City Health Center. Unlocking the Missing Link for Autoimmune Diseases | Queen City Health Center [Internet]. 2023 [cited 2024 Nov 3]. Available from:
73. Vatsalya V, Li F, Frimodig J, Gala KS, Srivastava S, Kong M, et al. Repurposing Treatment of Wernicke-Korsakoff Syndrome for Th-17 Cell Immune Storm Syndrome and Neurological Symptoms in COVID-19: Thiamine Efficacy and Safety, In-Vitro Evidence and Pharmacokinetic Profile. Front Pharmacol [Internet]. 2021 Mar 2 [cited 2024 Nov 3];11. Available from: https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2020.598128/full
74. Costantini A, Pala MI, Tundo S, Matteucci P. High-dose thiamine improves the symptoms of fibromyalgia. BMJ Case Rep. 2013 May 20;2013:bcr2013009019.
75. Costantini A, Nappo A, Pala MI, Zappone A. High dose thiamine improves fatigue in multiple sclerosis. BMJ Case Rep. 2013 Jul 16;2013:bcr2013009144.
76. MedlinePlus. Thiamine-responsive megaloblastic anemia syndrome: MedlinePlus Genetics [Internet]. [cited 2024 Nov 3]. Available from: https://medlineplus.gov/genetics/condition/thiamine-responsive-megaloblastic-anemia-syndrome/
77. Mount Sinai. Mount Sinai Health System. [cited 2024 Nov 3]. Vitamin B1 (Thiamine) Information | Mount Sinai – New York. Available from: https://www.mountsinai.org/health-library/supplement/vitamin-b1-thiamine
78. Antonio C. HIGH-D0SE THIAMINE (HDT) THERAPY for Parkinson’s Disease. 2024 [cited 2024 Nov 3]. HIGH-D0SE THIAMINE (HDT) THERAPY for Parkinson’s Disease. Available from: https://highdosethiamine.org/
79. Costantini A, Pala MI. Thiamine and Fatigue in Inflammatory Bowel Diseases: An Open-label Pilot Study | The Journal of Alternative and Complementary Medicine [Internet]. [cited 2024 Nov 3]. Available from: https://liebertpub.com/doi/full/10.1089/acm.2011.0840
80. Greco CM, Nakajima C, Manzi S. Updated Review of Complementary and Alternative Medicine Treatments for Systemic Lupus Erythematosus. Curr Rheumatol Rep. 2013 Nov;15(11):378.
81. Ahn H, Lee GS. Riboflavin, vitamin B2, attenuates NLRP3, NLRC4, AIM2, and non-canonical inflammasomes by the inhibition of caspase-1 activity | Scientific Reports [Internet]. [cited 2024 Nov 3]. Available from: https://www.nature.com/articles/s41598-020-76251-7
82. Suwannasom N, Kao I, Pruß A, Georgieva R, Bäumler H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int J Mol Sci. 2020 Jan 31;21(3):950.
83. Hill LJ, Williams AC. Meat Intake and the Dose of Vitamin B3 – Nicotinamide: Cause of the Causes of Disease Transitions, Health Divides, and Health Futures? Int J Tryptophan Res IJTR. 2017;10:1178646917704662.
84. White SD, Rosychuk RA, Reinke SI, Paradis M. Use of tetracycline and niacinamide for treatment of autoimmune skin disease in 31 dogs. J Am Vet Med Assoc. 1992 May 15;200(10):1497-500.
85. Surjana D, Damian DL. Nicotinamide in dermatology and photoprotection. Skinmed. 2011;9(6):360-5.
86. Chen AC, Damian DL. Nicotinamide and the skin. Australas J Dermatol. 2014 Aug;55(3):169-75.
87. Gale EA. Theory and practice of nicotinamide trials in pre-type 1 diabetes. J Pediatr Endocrinol Metab JPEM. 1996;9(3):375-9.
88. Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Mol Basel Switz. 2009 Sep 9;14(9):3446-85.
89. Nijhuis L, van de Wetering R. SAT0031 VITAMIN B3 (NAM) SUPPRESSES T CELL ACTIVATION IN AND PRODUCTION OF PRO-INFLAMMATORY CYTOKINES IN VITRO IN A DOSE DEPENDENT MANNER INDICATING THERAPEUTIC POTENTIAL FOR THE TREATMENT OF JIA | Annals of the Rheumatic Diseases [Internet]. [cited 2024 Nov 3]. Available from: https://ard.bmj.com/content/78/Suppl_2/1080.1
90. Rawji KS, Young AMH, Ghosh T, Michaels NJ, Mirzaei R, Kappen J, et al. Niacin-mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathol (Berl). 2020 May;139(5):893-909.
91. Chen C, Zhang W, Zhou T, Liu Q, Han C, Huang Z, et al. Vitamin B5 rewires Th17 cell metabolism via impeding PKM2 nuclear translocation. Cell Rep. 2022 Nov 29;41(9):111741.
92. Imami M. 3-[(2,4-dihydroxy-3,3-dimethylbutanoyl)amino]propanoic acid (Vitamin B5): Its Synthesis, Transformation into Coenzyme A and Role in Disease. UTSCs J Nat Sci. 2(1):102-15.
93. Minami Y, Hirabayashi Y, Nagata C, Ishii T, Harigae H, Sasaki T. Intakes of vitamin B6 and dietary fiber and clinical course of systemic lupus erythematosus: a prospective study of Japanese female patients. J Epidemiol. 2011;21(4):246-54.
94. Mikkelsen K, Dargahi N, Fraser S, Apostolopoulos V. High-Dose Vitamin B6 (Pyridoxine) Displays Strong Anti-Inflammatory Properties in Lipopolysaccharide-Stimulated Monocytes. Biomedicines. 2023 Sep 19;11(9):2578.
95. Du X, Yang Y, Zhan X, Huang Y, Fu Y, Zhang Z, et al. Vitamin B6 prevents excessive inflammation by reducing accumulation of sphingosine-1-phosphate in a sphingosine-1-phosphate lyase-dependent manner. J Cell Mol Med. 2020 Nov;24(22):13129-38.
96. Cheng CH, Chang SJ, Lee BJ, Lin KL, Huang YC. Vitamin B6 supplementation increases immune responses in critically ill patients. Eur J Clin Nutr. 2006 Oct;60(10):1207-13.
97. Giil LM, Midttun Ø, Refsum H, Ulvik A, Advani R, Smith AD, et al. Kynurenine Pathway Metabolites in Alzheimer’s Disease. J Alzheimers Dis JAD. 2017;60(2):495-504.
98. Bendich A, Cohen M. Vitamin B6 safety issues. Ann N Y Acad Sci. 1990;585:321-30.
99. Sedel F, Bernard D, Mock DM, Tourbah A. Targeting demyelination and virtual hypoxia with high-dose biotin as a treatment for progressive multiple sclerosis. Neuropharmacology. 2016 Nov;110(Pt B):644-53.
100. Isola S, Gammeri L, Furci F, Gangemi S, Pioggia G, Allegra A. Vitamin C Supplementation in the Treatment of Autoimmune and Onco-Hematological Diseases: From Prophylaxis to Adjuvant Therapy. Int J Mol Sci. 2024 Jul 2;25(13):7284.
101. Kodama M, Kodama T, Murakami M, Kodama M. Autoimmune disease and allergy are controlled by vitamin C treatment. Vivo Athens Greece. 1994;8(2):251-7.
102. Tam LS, Li EK, Leung VYF, Griffith JF, Benzie IFF, Lim PL, et al. Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: a double blind, placebo controlled pilot study. J Rheumatol. 2005 Feb;32(2):275-82.
103. Minami Y, Sasaki T, Arai Y, Kurisu Y, Hisamichi S. Diet and systemic lupus erythematosus: a 4 year prospective study of Japanese patients. J Rheumatol. 2003 Apr;30(4):747-54.
104. Watad A, Neumann SG, Soriano A, Amital H, Shoenfeld Y. Vitamin D and Systemic Lupus Erythematosus: Myth or Reality? Isr Med Assoc J IMAJ. 2016;18(3-4):177-82.
105. Lomarat W, Pakchotanon RR. OP0283 A Randomized Double-Blind Comparative Clinical Trials To Evaluate Efficacy of Vitamin D in Systemic Lupus Erythematosus (SLE) Patients | Annals of the Rheumatic Diseases [Internet]. [cited 2024 Nov 3]. Available from: https://ard.bmj.com/content/75/Suppl_2/165.2
106. Carvalho JF, Blank M, Kiss E, Tarr T, Amital H, Shoenfeld Y. Anti-vitamin D, vitamin D in SLE: preliminary results. Ann N Y Acad Sci. 2007 Aug;1109:550-7.
107. Lemke D, Klement RJ, Schweiger F, Schweiger B, Spitz J. Vitamin D Resistance as a Possible Cause of Autoimmune Diseases: A Hypothesis Confirmed by a Therapeutic High-Dose Vitamin D Protocol. Front Immunol. 2021;12:655739.
108. Cheng RZ. Understanding and Addressing Vitamin D Resistance: A Comprehensive Approach Integrating Genetic, Environmental, and Nutritional Factors [Internet]. Available from: https://orthomolecular.org/resources/omns/v20n13.shtml
109. Yap KS, Morand EF. Vitamin D and systemic lupus erythematosus: continued evolution. Int J Rheum Dis. 2015 Feb;18(2):242-9.
110. Tolentino M, Cho CC, Lyons JA. Photobiomodulation therapy (PBMT) regulates the production of IL-10 and IFN-Ɣ by peripheral blood mononuclear cells (PBMC) and CD4+ T cells isolated from subjects with Multiple Sclerosis (MS). J Immunol. 2019 May 1;202(1_Supplement):193.16.
111. Tolentino M, Cho CC, Lyons JA. Photobiomodulation (PBM) regulates nitric oxide (NO) production by peripheral blood mononuclear cells (PBMC) isolated from Multiple Sclerosis (MS) patients. J Immunol. 2020 May 1;204(1_Supplement):160.8.
112. Muili KA, Gopalakrishnan S, Meyer SL, Eells JT, Lyons JA. Amelioration of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice by Photobiomodulation Induced by 670 nm Light. PLOS ONE. 2012 Jan 24;7(1):e30655.
113. Extracorporeal photochemotherapy for the treatment of systemic lupus erythematosus. A Pilot study – Knobler – 1992 – Arthritis & Rheumatism – Wiley Online Library [Internet]. [cited 2024 Nov 3]. Available from: https://onlinelibrary.wiley.com/doi/10.1002/art.1780350311
114. Motta S, Monti M. Photodynamic therapy-a promising treatment option for autoimmune skin ulcers: a case report | Photochemical & Photobiological Sciences [Internet]. Nov. 1, 27 [cited 2024 Nov 3]. Available from: https://link.springer.com/article/10.1039/b711920h
115. Wang J, Zhao C, Kong P, Bian G, Sun Z, Sun Y, et al. Methylene blue alleviates experimental autoimmune encephalomyelitis by modulating AMPK/SIRT1 signaling pathway and Th17/Treg immune response. J Neuroimmunol. 2016 Oct 15;299:45-52.
116. Yin Y, Choi SC. Normalization of CD4+ T cell metabolism reverses lupus | Science Translational Medicine [Internet]. 2015 [cited 2024 Nov 3]. Available from: https://www.science.org/doi/10.1126/scitranslmed.aaa0835
117. Singer DS, Kohn LD, Zinger H, Mozes E. Methimazole prevents induction of experimental systemic lupus erythematosus in mice. J Immunol. 1994 Jul 15;153(2):873-80.
118. Reilly CM, Regna N, Mishra N. HDAC Inhibition in Lupus Models. Mol Med. 2011 May;17(5):417-25.
119. Weeding E, Sawalha AH. Deoxyribonucleic Acid Methylation in Systemic Lupus Erythematosus: Implications for Future Clinical Practice. Front Immunol [Internet]. 2018 Apr 24 [cited 2024 Nov 3];9. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2018.00875/full
120. Albano S, Gallicchio VS. Systemic Lupus Erythematosus & Stem Cell Therapy. Stem Cell Regen Med [Internet]. 2023 Jun 30 [cited 2024 Nov 3];7(1). Available from: https://www.scivisionpub.com/pdfs/systemic-lupus-erythematosus–stem-cell-therapy-2774.pdf
121. Li A, Guo F, Pan Q, Chen S, Chen J, Liu H feng, et al. Mesenchymal Stem Cell Therapy: Hope for Patients With Systemic Lupus Erythematosus. Front Immunol [Internet]. 2021 Sep 30 [cited 2024 Nov 3];12. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2021.728190/full
122. Yang Q, Liu Y. An Overview of the Safety, Efficiency, and Signal Pathways of Stem Cell Therapy for Systemic Lupus Erythematosus – Yang – 2021 – Stem Cells International – Wiley Online Library [Internet]. 2021 [cited 2024 Nov 3]. Available from: https://onlinelibrary.wiley.com/doi/10.1155/2021/2168595
123. Zare Moghaddam M, Mousavi MJ, Ghotloo S. Stem cell-based therapy for systemic lupus erythematous. J Transl Autoimmun. 2024 Jun 1;8:100241.
124. Yuan X, Sun L. Stem cell therapy in lupus. Rheumatol Immunol Res. 2022 Jun 1;3(2):61-8.
125. Sui W, Hou X, Che W, Chen J, Ou M, Xue W, et al. Hematopoietic and mesenchymal stem cell transplantation for severe and refractory systemic lupus erythematosus. Clin Immunol. 2013 Aug 1;148(2):186-97.
126. Cheng RZ. Integrative Low Carb/Orthomolecular Medicine for Autoimmune Diseases [Internet]. 2022 Sep 5. Available from: https://www.youtube.com/watch?v=noScK80HVMs
127. Cheng RZ. Reversing Hashimoto’s Thyroiditis with Orthomolecuar Medicine [Internet]. 2022. Available from: https://www.drwlc.com/blog/2022/05/20/reversing-hashimotos-thyroiditis-with-orthomolecular-medicine/
Nutritional Medicine is Orthomolecular Medicine
Orthomolecular medicine uses safe, effective nutritional therapy to fight illness. For more information: http://www.orthomolecular.org
Find a Doctor
To locate an orthomolecular physician near you: http://orthomolecular.org/resources/omns/v06n09.shtml
The peer-reviewed Orthomolecular Medicine News Service is a non-profit and non-commercial informational resource.
Editorial Review Board:
Albert G. B. Amoa, MB.Ch.B, Ph.D. (Ghana)
Seth Ayettey, M.B., Ch.B., Ph.D. (Ghana)
Ilyès Baghli, M.D. (Algeria)
Barry Breger, M.D. (Canada)
Ian Brighthope, MBBS, FACNEM (Australia)
Gilbert Henri Crussol, D.M.D. (Spain)
Carolyn Dean, M.D., N.D. (USA)
Ian Dettman, Ph.D. (Australia)
Susan R. Downs, M.D., M.P.H. (USA)
Ron Ehrlich, B.D.S. (Australia)
Hugo Galindo, M.D. (Colombia)
Gary S. Goldman, Ph.D. (USA)
William B. Grant, Ph.D. (USA)
Claus Hancke, MD, FACAM (Denmark)
Patrick Holford, BSc (United Kingdom)
Ron Hunninghake, M.D. (USA)
Bo H. Jonsson, M.D., Ph.D. (Sweden)
Dwight Kalita, Ph.D. (USA)
Felix I. D. Konotey-Ahulu, M.D., FRCP (Ghana)
Peter H. Lauda, M.D. (Austria)
Fabrice Leu, N.D., (Switzerland)
Alan Lien, Ph.D. (Taiwan)
Homer Lim, M.D. (Philippines)
Stuart Lindsey, Pharm.D. (USA)
Pedro Gonzalez Lombana, M.D., Ph.D. (Colombia)
Victor A. Marcial-Vega, M.D. (Puerto Rico)
Juan Manuel Martinez, M.D. (Colombia)
Mignonne Mary, M.D. (USA)
Joseph Mercola, D.O. (USA)
Dr.Aarti Midha M.D., ABAARM (India)
Jorge R. Miranda-Massari, Pharm.D. (Puerto Rico)
Karin Munsterhjelm-Ahumada, M.D. (Finland)
Sarah Myhill, MB, BS (United Kingdom)
Tahar Naili, M.D. (Algeria)
Zhiyong Peng, M.D. (China)
Isabella Akyinbah Quakyi, Ph.D. (Ghana)
Selvam Rengasamy, MBBS, FRCOG (Malaysia)
Jeffrey A. Ruterbusch, D.O. (USA)
Gert E. Schuitemaker, Ph.D. (Netherlands)
Thomas N. Seyfried, Ph.D. (USA)
Han Ping Shi, M.D., Ph.D. (China)
T.E. Gabriel Stewart, M.B.B.CH. (Ireland)
Jagan Nathan Vamanan, M.D. (India)
Andrew W. Saul, Ph.D. (USA), Founding Editor
Richard Cheng, M.D., Ph.D. (USA), Editor-In-Chief
Associate Editor: Robert G. Smith, Ph.D. (USA)
Editor, Japanese Edition: Atsuo Yanagisawa, M.D., Ph.D. (Japan)
Editor, Chinese Edition: Richard Cheng, M.D., Ph.D. (USA)
Editor, Norwegian Edition: Dag Viljen Poleszynski, Ph.D. (Norway)
Editor, Arabic Edition: Moustafa Kamel, R.Ph, P.G.C.M (Egypt)
Editor, Korean Edition: Hyoungjoo Shin, M.D. (South Korea)
Editor, Spanish Edition: Sonia Rita Rial, PhD (Argentina)
Editor, German Edition: Bernhard Welker, M.D. (Germany)
Associate Editor, German Edition: Gerhard Dachtler, M.Eng. (Germany)
Assistant Editor: Michael Passwater (USA)
Contributing Editor: Thomas E. Levy, M.D., J.D. (USA)
Contributing Editor: Damien Downing, M.B.B.S., M.R.S.B. (United Kingdom)
Contributing Editor: W. Todd Penberthy, Ph.D. (USA)
Contributing Editor: Ken Walker, M.D. (Canada)
Contributing Editor: Michael J. Gonzalez, N.M.D., Ph.D. (Puerto Rico)
Technology Editor: Michael S. Stewart, B.Sc.C.S. (USA)
Associate Technology Editor: Robert C. Kennedy, M.S. (USA)
Legal Consultant: Jason M. Saul, JD (USA)
Comments and media contact: editor@orthomolecular.org OMNS welcomes but is unable to respond to individual reader emails. Reader comments become the property of OMNS and may or may not be used for publication.
This article may be reprinted free of charge provided 1) that there is clear attribution to the Orthomolecular Medicine News Service, and 2) that both the OMNS free subscription link http://orthomolecular.org/subscribe.html and also the OMNS archive link http://orthomolecular.org/resources/omns/index.shtml are included.
Riordan Clinic | Orthomolecular.org
3100 N Hillside Ave
Wichita, Kansas 67219
United States
‘Long Vax’ Finally Enters Lexicon
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2024/04/05/covid-vaccine-long-vax.aspx
Analysis by Dr. Joseph Mercola April 05, 2024

Story at-a-glance
- Dr. Pierre Kory and Dr. Paul Marik are trying to get the word out that long vax is not only real but has disabled many Americans who were at the peak of health prior to getting a COVID jab
- At Kory’s long COVID clinic, 70% of the patients actually have long vax and reported their symptoms began “minutes, hours, days or several weeks” after receiving a COVID-19 shot
- Long vax symptoms are nearly identical to those of long COVID — the difference being that the long vax patients tend to be sicker, with more frequent small fiber neuropathy and dysautonomia
- A study by Yale scientists detailed long vax, which they called chronic post-vaccination syndrome, in 241 people
- Top reported symptoms include exercise intolerance, excessive fatigue, numbness, brain fog and neuropathy
An estimated 6.4% of U.S. adults have experienced symptoms of long COVID, a term used to describe a complex disorder that persists for three or more months after contracting COVID-19.1 While long COVID has been extensively covered in the media, millions more suffer from long vax — a condition with nearly identical symptoms to long COVID, but often even more severe.
New York pulmonologist Dr. Pierre Kory and Dr. Paul Marik, a critical care doctor formerly with Sentara Norfolk General Hospital in East Virginia, are part of the Front Line COVID-19 Critical Care Working Group (FLCCC). They’re trying to get the word out that long vax is not only real but has disabled many Americans who were at the peak of health prior to getting a COVID jab.2
At Long COVID Clinic, 70% of Patients Have Long Vax
Kory opened a tele-health practice that specializes in treating COVID disease, including long COVID. Kory says:3
“Long COVID, although a new name, is not a new disease. It meets the diagnostic criteria for a decades-old condition called myalgic encephalitis/chronic fatigue syndrome (ME/CFS).
The three symptom ‘pillars’ which lead to the diagnosis are fatigue, post-exertional malaise (PEM), and ‘brain fog’ (i.e. cognitive deficits ranging from word finding difficulties, short term memory loss, inability to focus/comprehend, and more rarely confusion or disorientation).
Although this triad is present in nearly every patient I see (rarely brain fog is missing), the patients also present with a ‘side menu’ of problems which can include sensory neuropathies, dysautonomia/POTS, motor neuropathies, abdominal issues, musculoskeletal complaints, and cranial symptoms (i.e tinnitus, vertigo, headaches, vision, hearing loss, smell loss, taste loss).
Many of my patients are debilitated and meet criteria for disability, despite the majority reporting being in the peak of health and functioning prior to the pandemic.”
Yet, Kory and colleagues quickly noticed that most of their patients reported their symptoms began “minutes, hours, days or several weeks”4 after receiving a COVID-19 shot. While many had also had COVID-19, only a small number tied their symptoms to the viral infection.
While the team initially called the condition post-COVID vaccine injury syndrome, they changed the diagnosis to “long vax” because the symptoms were so close to long COVID — the difference being that the long vax patients tended to be sicker, with more frequent small fiber neuropathy and dysautonomia, Kory said.5
Research Details Neuropathic Symptoms Following COVID-19 Jabs
Scientific studies detailing long vax symptoms continue to emerge. In one study from early in the pandemic, more than two-thirds of those reporting long COVID symptoms had negative antibody tests, suggesting at least some of them didn’t even have COVID-19.6 Meanwhile, many COVID jab recipients report long COVID-like symptoms.
As reported by Science magazine in 2022, “In rare cases, coronavirus vaccines may cause long COVID-like symptoms,”7 which can include (but is not limited to) brain fog, memory problems, headaches, blurred vision, loss of smell, nerve pain, heart rate fluctuations, dramatic blood pressure swings and muscle weakness. The feeling of “internal electric shocks” are also reported.
Also in 2022, a preprint study from the U.S. National Institutes of Health reported new neuropathic symptoms that began in 23 adults within one month of receiving a COVID-19 shot.8 All of the patients felt severe tingling or numbness in their faces or limbs, and 61% also experienced dizziness when standing up, intolerance to heat and heart palpitations.
When 12 of the patients had their nerve function tested, seven had less sweating in their hands and feet than normal, while six had a condition where their heart beats too fast when they stand up.9
The researchers also took skin samples from the lower legs of 16 patients. Among them, 31% showed signs that the small nerves in the skin were not as dense as they should be, which can indicate nerve damage. Another 13% were on the border of being considered damaged, and 19% had swollen nerve fibers. When five of the samples were evaluated more closely, signs of an immune reaction in the blood vessels were detected.10
Further, while electrical tests on the nerves were normal for most participants, 52% showed clear signs of damage to the small nerves that can be felt but not easily seen. The study shows that after getting the COVID-19 shot, a range of symptoms related to nerve damage is possible, which might be caused by an immune system reaction.

Save This Article for Later – Get the PDF Now
Yale Scientists Detail Long Vax Symptoms
A study by Yale scientists, including Dr. Harlan Krumholz of Yale School of Medicine in New Haven, Connecticut, also shed light on long vax, which they described as chronic post-vaccination syndrome, or PVS.11 In a study of 241 people who reported PVS after an mRNA COVID-19 shot, the median time from the jab to the onset of symptoms was three days, with symptoms continuing for 595 days. The five most common symptoms included:12
- Exercise intolerance (71%)
- Excessive fatigue (69%)
- Numbness (63%)
- Brain fog (63%)
- Neuropathy (63%)
In the week before the survey was completed, patients reported a range of additional symptoms highlighting the mental toll the condition takes. The symptoms required a median of 20 interventions for treatment and included:13
| Feeling unease (93%) | Fearfulness (82%) |
| Overwhelmed by worries (81%) | Feelings of helplessness (80%) |
| Anxiety (76%) | Depression (76%) |
| Hopelessness (72%) | Worthlessness (49%) |
“In this study,” the researchers explained, “individuals who reported PVS after COVID-19 vaccination had low health status, high symptom burden, and high psychosocial stress despite trying many treatments. There is a need for continued investigation to understand and treat this condition.”14
Even a 2021 study reported a series of patients who experienced new autoimmune conditions — or flare-ups of existing autoimmune disease — following mRNA COVID-19 shots,15 highlighting the importance of careful research into the ongoing health risks.
Will Long Vax Be Censored?
It’s hopeful that scientific on long vax is reaching medical journals and getting some media coverage.16 But Kory and Marik are concerned it could reach a similar fate as other COVID shot coverage during the pandemic.
“The concern is that our findings, Krumholz’s study, and any reports of adverse events from COVID-19 vaccination, will be subject to the same institutional censorship we saw throughout the pandemic. Suppressing this information risks creating an even bigger disaster,” they told The Hill,17 referring to a potential epidemic of autoimmune diseases that could occur as a result.
“America’s health agencies need to snap into action to help study this problem so we can better understand and treat these conditions. Unfortunately, there doesn’t seem to be much hope of this happening,” Kory and Marik wrote. “The National Institutes of Health is fixated on studying the effect of Paxlovid, an antiviral COVID treatment, to treat long COVID and long vax, despite it having no proven effect on autoimmune disease.”18
Further, Kory explains that while major medical centers and hospitals across the U.S. have opened long COVID clinics, the treatments they offer are largely ineffective, and they often gas-lit long vax patients who tried to get help:19
“[F]or most of 2022 into 2023, those centers consistently gas-lit the Long Vax patients who presented to those clinics. Gaslighting of medical injuries is the well-described inability for physicians to recognize or accept when their own treatments (i.e the mRNA vaccines) cause harm …
The stories my patients would tell me of the care they received included what I would describe as abuse or insults from the treating physicians when the patients tried to convince them that the vaccines were the cause. These stories still make my blood boil and have estranged many of my patients from ‘the system.’ I believe the gaslighting responses have lessened somewhat but I don’t really know how much.
What angered me even further is that the health agencies only directed funding at long COVID and the medical literature and media only referred to sufferers as having long COVID. The contribution of the gene therapy vaccines are consistently ignored.”
Is Long Vax Behind the Explosion of Disability Claims?
Kory believes that long vax, and to a lesser extent long COVID, are behind the explosion of disability claims that have occurred since COVID-19 shots rolled out.20
Data compiled by former BlackRock analyst and fund manager Edward Dowd revealed a sobering glimpse into the true carnage that occurred at the hands of the COVID-19 shot campaign,21 and its results are striking. It revealed the following estimated human and economic costs:22
Human cost:
- 26.6 million injuries
- 1.36 million disabilities
- 300,000 excess deaths
Economic cost:
- Total: $147.8 billion
- Injuries: $89.9 billion
- Disabilities: $52.2 billion
- Excess deaths: $5.6 billion
What’s more, this data is from the employed population, aged 16 to 64 — a typically healthy crowd. To put this into perspective, John Leake writes on Courageous Discourse, “Note that this death count in one year is 5.2 times the number of men killed in ten years of combat in Vietnam.”23
Help for Long Vax Symptoms
As long vax and its symptoms become increasingly recognized, it will hopefully lead to increased access to effective treatments. If you’re experiencing symptoms, it’s important to find a holistic health care practitioner who’s familiar with long vax and how to treat it. You can also access FLCCC’s I-RECOVER24 guide,25 which offers step-by-step instructions on how to treat reactions from COVID-19 injections.26
I also summarized strategies to optimize mitochondrial health if you’re suffering from long COVID or long vax, with a focus on boosting mitochondrial health. To allow your body to heal you’ll want to minimize EMF exposure as much as possible. Your diet also matters, as the cristae of the inner membrane of the mitochondria contains a fat called cardiolipin, the function of which is dependent on the type of fat you get from your diet.
The type of dietary fat that promotes healthy cardiolipin is omega-3 fat, and the type that destroys it is omega-6, especially linoleic acid (LA), which is highly susceptible to oxidation. So, to optimize your mitochondrial function, you want to avoid LA as much as possible and increase your intake of omega-3s.
Primary sources of LA include seed oils used in cooking, processed foods and restaurant foods made with seed oils, condiments, seeds and nuts, most olive oils and avocado oils (due to the high prevalence of adulteration with cheaper seed oils). Animal foods raised on grains, such as conventional chicken and pork, are also high in LA.
Another major culprit that destroys mitochondrial function is excess iron — and almost everyone has too much iron. You can learn more about the health risks of excess iron in my interview with Christy Sutton, D.C. The most effective way to lower your iron is to donate blood two to four times a year.
Copper is also important for energy metabolism, detoxification and mitochondrial function, and copper deficiency is common. Other strategies include sun exposure and near-infrared light therapy, NAD+ optimizers and methylene blue, which can be a valuable rescue remedy. By improving your mitochondrial function and restoring the energy supply to your cells, you’ll significantly increase your odds of reversing the problems caused by the jab or the virus.
- 1 U.S. CDC, MMWR February 15, 2024 / 73(6);135–136
- 2, 3, 4, 5, 19, 20 Pierre Kory’s Medical Musings March 8, 2024
- 6 TNR December 8, 2022
- 7 Science January 20, 2022
- 8, 9, 10 medRxiv May 17, 2022
- 11, 12, 13, 14 medRxiv November 10, 2023
- 15 Int Immunopharmacol. 2021 Oct:99:107970. doi: 10.1016/j.intimp.2021.107970. Epub 2021 Jul 10
- 16, 17, 18 The Hill March 6, 2024
- 21 Phinance Technologies, The Vaccine Damage Project March 2023
- 22 Zero Hedge March 28, 2023
- 23 Substack, Courageous Discourse March 29, 2023
- 24, 25 FLCCC Alliance, I-RECOVER
- 26 FLCCC Alliance, I-RECOVER, Post-Vaccine Treatment Guide
Canola Oil Proven to Destroy Your Body and Mind
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2024/03/18/canola-oil-health-effects.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola March 18, 2024

STORY AT-A-GLANCE
- According to AARP, 93% of Americans are concerned with their brain health, but few understand how to protect it
- Research demonstrates canola oil led to significant declines in working memory and has a significant impact on weight management
- Canola oil is not healthy fat vital to your brain; it is manufactured from genetically engineered rapeseed plants altered to reduce levels of erucic acid toxic to humans and processed through several chemical baths before being bleached
- Healthier options include pastured, organic butter, virgin coconut oil, ghee (clarified butter) and lard for cooking and olive oil for non-cooking purposes
According to a study by AARP,1 93% of Americans are concerned with their brain health, but very few understood some of the natural strategies they could use to improve it. Contrary to popular belief, your brain function and cognitive performance do not have to decline with age. There are steps you can take that influence your memory, processing, executive functions and more.
Even if you are already in your “golden years,” simple changes may prompt brain health for the better. For instance, where once it was believed that neurons were only generated early in life, scientists now know that neurogenesis (generation of new neurons) continues into adulthood.2 Exactly what influences the rate of new neuron growth is still being explored, as are other factors that play a role in brain health.
Research, for instance, has uncovered damage canola oil consumption triggers in your brain and the effect this may have on your memory and learning ability.3 The study, published in the journal Nature, also found the consumption of canola oil increased weight gain.
Canola Oil Negatively Affects Brain Health and Weight Management
The study was led by researcher Dr. Domenico Praticò from Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania. Praticò commented to the Los Angeles Times that canola oil is perceived by many to be healthy — a widespread misconception:4
“Canola oil is appealing because it is less expensive than other vegetable oils, and it is advertised as being healthy. Very few studies, however, have examined that claim, especially in terms of the brain.”
Researchers used an animal model to evaluate the effect canola oil has on the brains of mice genetically engineered to develop Alzheimer’s disease.5 Canola oil developed a reputation of being healthy when doctors began warning people to reduce their saturated fat intake and consume vegetable oils instead. Canola has the lowest percentage of saturated fat of all commonly used vegetable oils and is relatively inexpensive, but is actually one of the worst oils for your health.
Canola oil is often used in homes and restaurants for baking, sautéing, frying and other forms of cooking, with consumers being deceived into believing it’s better for them than saturated fats. The mice were split into two groups; one group was fed the usual chow and the second group was fed chow with the human equivalent of 2 teaspoons of canola oil per day.
At the end of the experimental six months, researchers observed that the mice eating chow laced with canola oil were significantly heavier than the mice that did not eat canola oil. Additionally, the mice who had eaten canola oil demonstrated significant declines in working memory together with a decreased level of post-synaptic density protein-94, a marker of synaptic integrity. The researchers found canola oil had a negative effect on health and concluded:6
“Taken together, our findings do not support a beneficial effect of chronic canola oil consumption on two important aspects of AD [Alzheimer’s disease] pathophysiology which includes memory impairments as well as synaptic integrity.”
Your Brain Needs Healthy Fats
The same researchers used a similar model to evaluate the effects of olive oil on the brain function of mice.7 In that study,8,9 neither group was heavier than the other, and the mice fed chow enriched with extra-virgin olive oil performed significantly better on testing that evaluated the animals’ working memory, spatial memory and ability to learn.
The brain tissue of these mice, genetically engineered to develop Alzheimer’s disease as they age like the mice in the featured study, also revealed dramatic differences. The mice fed olive oil demonstrated preserved synaptic integrity and an increase in nerve cell autophagy, ultimately responsible for a reduction in amyloid plaques common in the brain of those with Alzheimer’s disease.10
Healthy fat is an essential component of the structure of your brain, which is composed of nearly 60% fat.11 It should come as no surprise that your brain needs quality fat to function optimally. Although your brain is a small part of your complete bodyweight, it uses 20% of your metabolic energy. Essential fatty acids are required but cannot be synthesized in your body, and so must come from dietary sources.
Most people get well over what is needed of omega-6 fats, which are found in most vegetable oils, and not nearly enough omega-3 fats. One omega-3 fat, DHA, has been linked with the growth of your retina and visual cortex during development,12 visual acuity and reduction in depression. Research has found those suffering from attention deficit hyperactivity disorder (ADHD) have lower levels of DHA, and DHA may play a role in neuroprotection.
Unlike the highly damaged fats in vegetable oil, saturated fat is the optimal “clean” fuel for your brain and is one of the main components of brain cells. As such, it’s excellent for brain health, with one study demonstrating that those who ate more saturated fat reduced their risk of developing dementia while those who favored carbohydrates had a significantly increased risk.13
To maintain optimal brain function, you need high-quality, undamaged omega-3s and omega-6 along with antioxidants to protect them from oxidation — not processed vegetable oils like canola oil. In summary, processed vegetable oils are bad for your brain health for a number of reasons, including the following:
- They are loaded with damaged omega-6 fatty acids without protective antioxidants
- They strip your liver of glutathione, which produces antioxidant enzymes, which further lowers your antioxidant defenses
- Most vegetable oils are made with genetically engineered (GE) crops designed to resist herbicides like glyphosate. As such, they may be more contaminated with glyphosate than non-GE crops, and glyphosate has been shown to disrupt the tight junctions in your gut and increase penetration of foreign invaders, especially heated proteins, which can cause allergies

Save This Article for Later – Get the PDF Now
Vilification of Healthy Fats Has Contributed to Rising Rates of Disease
Defaming healthy fats over the past decades has contributed to a rising rate of disease. Although healthy fats are used as fuel and leave you feeling full, many turned to eating carbohydrates when fats were discouraged. Carbs are metabolized and burned quickly, using insulin to usher blood glucose into the cell.
However, carbs trigger insulin resistance over time and increase the potential for crashing blood sugar levels two to three hours after a meal, leaving you hungry once again and increasing your food intake. This one mechanism increases your risk for obesity, which in turn increases your potential risk for insulin resistance, Type 2 diabetes, cardiovascular disease and stroke.
In a time when healthy saturated fats and dietary cholesterol were publicly slandered, Canada developed an alternative oil that met with the approval of the American Heart Association (AHA) — canola oil.14 Now sitting in the first position of recommended oils for healthy cooking on the AHA website, author Praticò had this to say about the results of his canola oil study:15
“Amyloid-beta 1-40 neutralizes the actions of amyloid 1-42, which means that a decrease in 1-40, like the one observed in our study, leaves 1-42 unchecked. In our model, this change in ratio resulted in considerable neuronal damage, decreased neural contacts, and memory impairment.”
In other words, consuming canola oil may increase your risk of developing Alzheimer’s disease, as the oil decreases the production of a protein that protects your brain against neuronal damage and cognitive impairment.
Toxicity of Canola Oil May Result From the Seed, Source or Processing
This short video shows you the conditions under which canola oil is manufactured and produced, including the deplorable number of chemicals and bleaches added to the product to achieve the clear liquid you see on your grocery store shelves. Just the way the oil is processed should be enough to encourage you to steer clear of consuming the product. But the risk associated with canola oil doesn’t stop with processing.
The canola plant was developed from rapeseed plants by Agriculture and Agri-Food Canada and the University of Manitoba using plant breeding techniques. In fact, the Canola Council of Canada calls the development, “Canada’s greatest agricultural success story.”16 Rapeseed oil was originally used as a motor lubricant during World War II.17 Once the war ended, demand plummeted and Canada began an intensive program to make the product edible.
Before it could be ingested the erucic acid and glucosinolates had to be bred out of the plant, as they are dangerous to human health.18 By the late 1970s, both chemicals were reduced to lower levels, and the plant was officially accepted as consumable. In the 1980s, research focused on shelf stability of the oil, animal diets and gaining a wider consumer acceptance.
By 2012, nearly all low-erucic acid rapeseed plants were genetically engineered to increase yield. Today, what began as a motor lubricant is now one of Canada’s most profitable crops.
The featured study evaluated the effect of canola oil on brain function without identifying which characteristic of the product triggers the problems. However, as most canola oil is produced from GE seeds, using plants originally unfit for human consumption and taken through a process that injects multiple chemicals and bleaches, it isn’t surprising the study was so conclusive.
Genetic Engineering Raises Health Risk With Each GE Food Consumed
This documentary details what happens when we use GE foods. Scientists are only beginning to uncover the long-term effects of splicing the genes of one living creature into another or developing a plant immune to the effects of herbicides.
However, some companies are not convinced by independently funded research and have relied on information from organizations such as the American Medical Association (AMA) and World Health Organization (WHO), which claim there is no credible evidence that GE foods are unsafe. However, even WHO admits:19
“Different GM [genetically modified] organisms include different genes inserted in different ways. This means that individual GM foods and their safety should be assessed on a case-by-case basis and that it is not possible to make general statements on the safety of all GM foods.”
In 2015, the European Commission decided it was in the best interest of their citizens to say “no” to genetically modified organisms (GMOs) within their borders, and all 28 countries required labeling of foods containing GMO products.20 This is in stark contrast to the U.S., where most canola grown is GE21 and products created from it not labeled as such.
Healthy Cooking Options
Cooking with nearly all vegetable oils is problematic as they don’t tolerate high heat. Healthier options for cooking include pastured, organic butter, virgin coconut oil, ghee (clarified butter) and lard. Olive oil and sesame oil add wonderful flavor and healthy fats to your foods, but they have very low smoke points and should be used unheated in salad dressings or drizzled over meats or vegetables for flavor.
Boosting Brain Health Naturally
It is never too late to support your neurological health. Remember, even small changes you make each day reap big rewards over time. Seek to change your habits consistently and persistently to support your memory, cognitive function and ultimately your enjoyment of everyday life. Here are several strategies you may use to improve your brain health:
| Vitamin D — There are strong links between low levels of vitamin D in Alzheimer’s patients and poor outcome on cognitive testing. Optimal vitamin D levels may protect brain cells by increasing the effectiveness of the glial cells in restoring damaged neurons. Additionally, vitamin D has anti-inflammatory properties. |
| Carotenoids — These antioxidant compounds are found most often in orange colored vegetables, such as sweet potatoes and carrots. Some carotenoids, such as lutein and zeaxanthin, are found in dark green vegetables, namely kale and spinach (as well as egg yolks). Lutein and zeaxanthin are best known for the role they play in vision health, but accumulating evidence suggests they play a role in cognitive health as well by enhancing neural efficiency.22 |
| Probiotics — You are likely familiar with the importance of probiotics for your gut health but may not know of the role they play in your cognitive health. Certain beneficial bacterial strains, such as those found in fermented foods, have a positive effect on your brain function.
In a study by the University of California Los Angeles, scientists found women who regularly consumed beneficial bacteria via yogurt experienced changes in multiple areas of their brain, including those related to sensory processing, cognition and emotion.23 |
| Exercise — Physical activity produces biochemical changes that strengthen and renew not only your body but also your brain — particularly areas associated with memory and learning. |
| Diet — Reducing overall calorie and carbohydrate consumption, while increasing healthy fats, has a powerful effect on your brain health. Beneficial health-promoting sources of healthy fats that your body — and your brain in particular — needs for optimal function include organic grass fed raw butter, olives, organic virgin olive oil and coconut oil, nuts like pecans and macadamia, free-range eggs, wild Alaskan salmon and avocado, for example.
Increasing your omega-3 fat intake and reducing consumption of damaged omega-6 fats (i.e., processed vegetable oils) in order to balance your omega-3-to-omega-6 ratio also has a significant benefit for your brain. |
| Sleep — Sleep not only is essential for regenerating your physical body, but imperative for reaching new mental insights and being able to see new creative solutions to old problems. Sleep removes the blinders and helps “reset” your brain to look at problems from a different perspective.
Research from Harvard indicates that people are 33% more likely to infer connections among distantly related ideas after sleeping,24 but few realize that their performance has actually improved. Sleep is also known to enhance your memories and help you “practice” and improve your performance of challenging skills. In fact, a single night of sleeping only four to six hours can impact your ability to think clearly the next day. |
- 1 AARP, January 20, 2015
- 2 Brain Aging: Models, Methods and Mechanisms
- 3, 6 Nature, 2017;7(17134)
- 4, 5 Los Angeles Times, December 11, 2017
- 7 Science Daily, June 21, 2017
- 8 Ann Clin Transl Neurol. 2017 Jun 21;4(8):564-574
- 9, 10 EurekAlert! June 21, 2017
- 11, 12 ACTA Neurology Taiwan, 2009; 18:231
- 13 Journal of Alzheimer’s Disease, 2012;32(2): 329
- 14 American Heart Association, Healthy Cooking Oils
- 15 Forbes, December 11, 2017
- 16 Canola Council of Canada
- 17 Small Footprint Family, The Inconvenient Truth About Canola Oil
- 18 Canola History, Good as Gold
- 19 World Health Organization, Food, genetically modified
- 20 Library of Congress, Restrictions on genetically modified organisms
- 21 Agricultural Marketing Resource Center, Canola Profile February 2022
- 22 Journal of International Neuropsychological Society, 2016; 23(1):11
- 23 Gastroenterology. 2013 Jun;144(7):1394-1401.e4
- 24 New York Times September 27, 2008
Latest Additions to Long COVID Recovery Protocol
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2024/02/24/long-covid-recovery-protocol.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola February 24, 2024
STORY AT-A-GLANCE
- The Front Line COVID-19 Critical Care Alliance has updated its treatment protocols for long COVID and post-jab injuries, adding time-restricted eating (TRE) and photobiomodulation (PBM), using either the sun or near-infrared sauna
- The primary benefit of TRE for these conditions is its ability to optimize your mitochondrial function, which is key for recovery from any illness or disease. As a general rule, I recommend compressing your eating window to between six and eight hours, and fasting for the remaining 16 to 18 hours each day
- Near-infrared light triggers production of melatonin in your mitochondria. Melatonin is one of the most important antioxidant molecules in your body. Aside from having direct antioxidant effects, it also stimulates the synthesis of glutathione and other important antioxidants
- Fifty-three percent of sunlight is near-infrared. Sauna Space also makes near-infrared sauna bulbs that mimic the sun, providing 39% near-infrared light, but no UV
- You can further optimize your mitochondria by combining PBM with methylene blue. This combination has been shown to provide neuroprotective benefits. It can also address the chronic fatigue that is so common in long COVID and post-jab injuries
The video above features the Front Line COVID-19 Critical Care Alliance’s (FLCCC) weekly update for October 26, 2022, in which I reviewed the benefits of two of the latest additions to the FLCCC’s long COVID recovery protocol,1 and their post-jab recovery protocol:2 time-restricted eating (TRE) and photobiomodulation using either the sun or a near-infrared sauna. Leading the discussion are FLCCC cofounders Dr. Paul Marik and Dr. Pierre Kory.
Time-Restricted Eating (TRE)
The vast majority of people eat across 12 hours or more, which is a recipe for metabolic disaster. Health statistics bear this out. In July 2022, the Journal of the American College of Cardiology3 posted an update on the metabolic fitness or flexibility of the American population.
Metabolic fitness includes things like blood glucose and blood sugar, blood pressure and weight, and metabolic flexibility refers to your body’s ability to seamlessly transition between burning fat and carbohydrates as your primary fuel.
In 2016, 12.2% of Americans were considered metabolically fit.4 Two years later, in 2018, only 6.8% of U.S. adults had optimal cardiometabolic health.5 That was four years ago, so today, that ratio is probably even lower.
I believe at least 95% of Americans are by now metabolically unhealthy, which means some 19 out of 20 people would benefit from TRE, as it’s one of the easiest yet most powerful interventions for reducing insulin resistance, restoring metabolic flexibility and losing excess body fat.
As it pertains to long COVID and post-jab recovery, the primary benefit of TRE is its ability to optimize your mitochondrial function, which is key for recovery from any illness or disease.
As a general rule, I recommend compressing your eating window to between six and eight hours, and fasting for the remaining 16 to 18 hours each day. The timing of that eating window is important though.
You want to avoid eating first thing in the morning (wait at least two or three hours) and you want to avoid eating right before bed. Ideally, have your last meal at least three hours or more before bedtime. So, to give you an example, you could eat all your meals between 10 a.m. and 6 p.m., or 11 a.m. and 5 p.m.
Near-Infrared Exposure and Melatonin
Another important mitochondrial tool is strategic use of near infrared light and its impact on melatonin, which is one of the most important antioxidant molecules in your body. Aside from having direct antioxidant effects, it also stimulates the synthesis of glutathione and other important antioxidants like superoxide dismutase and catalase.
While commonly thought of as something produced only in the pineal gland of your brain, which is produced in response to darkness, it only accounts for 5% of the melatonin in your body. A couple of years ago, Dr. Russel Reiter published a groundbreaking paper explaining that the vast majority, 95%, is actually made within the mitochondria inside your cells,6 where it is produced in response to near-infrared light, which could be from the sun or near-infrared sauna bulbs.
Considering melatonin combats oxidative damage, it makes sense that most of it is made in your mitochondria, because that’s precisely where a majority of the oxidative damage occurs — in the mitochondrial electron transport chain. So, it’s a truly phenomenal system. The key that makes this system work, however, is exposure to near-infrared light. To dive deeper into this topic, see my interview with Reiter.
This is one of the reasons why exposing as much bare skin as possible to the sun for an hour a day is my No. 1 recommendation to optimize your health. And it’s free. As you can see in the graph below, 52% of the sun’s rays are near-infrared.
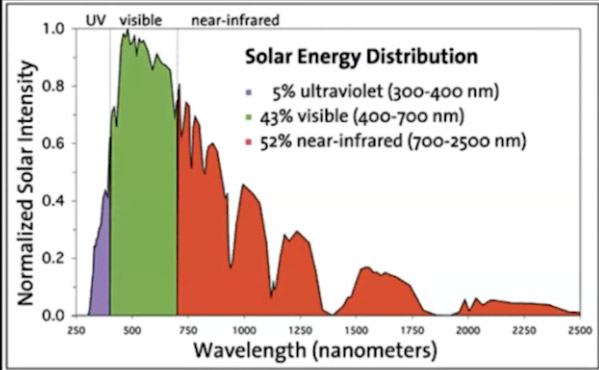

Download this Article Before it Disappears
Concerning Skin Cancer
As noted by Marik, many shy away from sun exposure as a source of UV and near IR for fear of skin cancer. This is yet another example of medical propaganda. The fact is the sun is a nutrient that will actually decrease your risk of both skin cancer and internal cancers, provided you avoid sun burn.
Another little-known fact is that the fats in your diet can, to a significant degree, dictate how predisposed you are to burning and sun-related skin damage. The key is to limit omega-6 fats in your diet. That means avoiding processed foods, foods cooked in seed oils, and animal foods raised on seed oils and/or grains, such as chicken and pork.
Omega-6 seed oils are primarily linoleic acid (LA), a polyunsaturated fat that is highly susceptible to oxidative damage when exposed to a variety of stressors. What’s more LA can remain embedded in your cell membranes and tissues for up to seven years, all the while wreaking havoc and causing damage through oxidative stress.
As it pertains to sun exposure, the LA embedded in your cell membranes gets activated by the exposure to the sun, which contributes to skin damage. Once you drastically reduce or eliminate LA from your diet, your risk of sunburn will be dramatically reduced over time, as will your risk of skin cancer.
General Benefits of Photobiomodulation (PBM)
Light in the red and infrared range has several important health benefits, including:
| Increasing mitochondrial ATP production | Increasing heat shock proteins, which help proteins maintain their three-dimensional structure and refold misfolded proteins |
| Activating cell stress responses | Increasing autophagy |
| Reducing inflammation | Speeding up wound healing |
Near-infrared light specifically, which is invisible to the naked eye, can penetrate 5 to 7 inches into your body, into your subcutaneous tissue, muscle tissue, bone and even interior tissues as you can see by the graphic below. This is in stark contrast to far IR that most saunas use, which penetrates less than half an inch.

This is what makes near-infrared saunas so useful, as you can boost melatonin production throughout your entire body, including your organs, thereby detoxing and healing them. In comparison, far-infrared penetrates only 1 to 2 millimeters, yet far-infrared is, unfortunately, what most commercially available electrical saunas use.
General Benefits of Sauna Therapy
Research has demonstrated sauna use can:
| Improve cardiovascular fitness and lower your risk of death from cardiovascular disease, stroke and heart attack |
| Lower your blood pressure |
| Lower your risk of dementia |
| Improve your mood and mental health, and reduce symptoms of depression, in part by sensitizing opioid receptors |
| Strengthen your immune function |
| Reduce all-cause mortality |
| Improve athletic endurance |
| Reduce inflammation by lowering c-reactive protein, and increasing IL-10 and IL-6 (aka, myokine), and activating Nrf2 |
| Activate and replenish stem cells |
| Improve fasting glucose and insulin sensitivity |
| Reduce the stress hormone cortisol7 |
All of these benefits occur in a dose-dependent manner, so the more frequent your sauna use, the more robust your benefits will be. For example, using the sauna two to three times a week has been shown to reduce your risk of cardiac death by about 22% compared to once-a-week use, whereas those who use it seven times a week lower their risk by 63%.
Similarly, those who use it four to seven times a week have a 40% lower all-cause mortality risk than those who use it only once a week. To learn more about these and other health benefits of sauna therapy, see “The Stunning Health Benefits of Sauna Therapy.”
Different Saunas Provide Different Sets of Benefits
Many of the benefits listed above are related to the heat exposure alone, and for heat, it doesn’t much matter what type of sauna you use: a traditional thermal Finnish sauna, a far-infrared sauna or a near-infrared one. Although one needs to be careful as many far IR saunas simply do not get hot enough to generate heat shock proteins and detox.
Near-infrared saunas have benefits that you simply cannot get with the others. Below is a simple chart showing the differences in benefits between the three primary types of saunas available in the U.S.

As you can see, all will provide detoxification to some degree (although the detox provided by far-infrared is questionable, seeing how it barely penetrates your tissue. Far-infrared saunas also oftentimes cannot get hot enough for efficient detox, which requires you to sweat profusely). Detoxification is something just about every person needs, as we’re surrounded by toxins and take them in with both food and water.
Unstructured proteins tend to aggregate, and these aggregates can then form plaques in your vascular system or brain, contributing to neurodegenerative diseases and cardiovascular problems. Heat shock proteins serve a really crucial role in keeping these kinds of problems at bay.
All three will also generate heat shock proteins to some degree. As mentioned earlier, heat shock proteins are important because they refold misfolded proteins and all of us have misfolded proteins.
Unstructured proteins tend to aggregate, and these aggregates can then form plaques in your vascular system or brain, contributing to neurodegenerative diseases and cardiovascular problems, so heat shock proteins serve a really crucial role in keeping these kinds of problems at bay.
Again, there’s a question mark by far-infrared, as many far-infrared saunas can’t get hot enough. Near-infrared saunas also are nowhere near as hot as the Finnish-style sauna, but they don’t need to be, due to the extreme penetration. You’ll sweat profusely and activate heat shock proteins even though the air temperature is lower than a Finnish thermal sauna.
You can determine if your sauna is hot enough for you by measuring your temperature with an oral thermometer. It should be around 101 to 103 degrees Fahrenheit as you finish your sauna. You could also weigh yourself before and after the sauna and you should lose between 2 and 4 pounds of water (sweat). This would be one pint to one quart of water.
Next on the list, melatonin production. This can only be achieved in a near-infrared sauna, as melatonin is not produced in response to mere heat or light in the far-infrared range.
The Importance of an EMF-Free Sauna
Lastly, there’s the issue of adverse health effects of electromagnetic fields (EMF). I published a book about this in early 2020, called “EMF*D.” Virtually no far-infrared saunas on the market are truly EMF-free, even when advertised as such. The reason for this is because EMFs include both electric and magnetic fields, and most saunas address only one of these.
If you’re using a wood-burning Finnish sauna, it will of course be EMF-free, but almost no one has that in the U.S. Most Finnish-style saunas sold here use electrical heaters and emit electrical fields. When you’re detoxing, you want to be in a parasympathetic environment. When you’re exposed to EMFs, it activates your sympathetic nervous system, which impairs your ability to detox properly.
The near-infrared Sauna Space sauna that I use and recommend has no EMFs. And, again, while traditional and some FIR saunas can provide outstanding benefits in detox and heat shock protein generation, neither of them provide the light wavelengths to generate PBM benefits.
What Make Sauna Space’s Bulbs so Unique
Sauna Space uses incandescent heat lamps (shown in the photo further below) that produce the majority of the heat as mid-infrared, plus a significant percentage of near- and mid-infrared frequencies that provide PBM benefits. The spectral distribution of these bulbs is shown in the following graph.
As mentioned, 53% of sunlight is near-infrared, and the Sauna Space bulbs mimic sunlight with 39% of the light being in the near-infrared range. (They do not have any UV light, however, so you will not make vitamin D. The bulbs only mimic sunlight in terms of their infrared distribution.) This is vastly superior to the average red heat lamp (graph below), in which only 14% is near-infrared.
In “Near-Infrared Sauna Therapy — A Key Biohack for Health,” I provide instructions for how you can create your own near-infrared sauna using the Sauna Space near-infrared bulbs.
Sauna Space is the only company that I know of that makes these kinds of bulbs, so they’ve really cornered the market, at least for now. Using these, you’ll be able to make the absolute best, most effective sauna there is, as you’re getting both the standard sauna benefits and the PBM benefits, and none of the EMF hazards.

Optimizing Photobiomodulation With Methylene Blue
If you want to further optimize your mitochondria, you can combine PBM (the near-infrared light) with methylene blue. A 2020 paper8 in Translational Neurodegeneration reviews the benefits of this combination, specifically as it refers to neuroprotection. I believe this combination can also be very valuable in the treatment of long COVID and in post-jab recovery, many symptoms of which are neurological in character.
In 1890, the German physician Dr. Paul Ehrlich published a study showing methylene blue could effectively treat malaria. Interestingly, many of the most useful drugs for COVID are antimalarial and antiparasitic drugs such as hydroxychloroquine. Well, methylene blue is the parent molecule to chloroquine.
Methylene blue can be particularly useful for addressing the fatigue that is so common in long COVID. You can learn more in “The Surprising Health Benefits of Methylene Blue,” in which I interview Francisco Gonzalez-Lima, Ph.D., who has spent many years studying this drug.
In closing, you can find the latest, updated protocols for long COVID9 and post-jab injuries10 on the FLCCC’s website. These protocols are continually updated as the team learns more.
- 1, 9 FLCCC Alliance, I-RECOVER Long COVID Treatment
- 2, 10 FLCCC Alliance, I-RECOVER Post-Vaccine Treatment Protocol
- 3 Journal of the American College of Cardiology July 2022; 80(2): 138-151
- 4, 5 Metab Syndr Relat Disord February 2019; 17(1): 46-52
- 6 Physiology February 5, 2020
- 7 YouTube The Science & Health Benefits of Deliberate Heat Exposure | Huberman Lab Podcast #69
- 8 Translational Neurodegeneration 2020; 9: 19
Liposomal Carnosine Is Essential for Detoxing Linoleic Acid
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2024/02/05/liposomal-carnosine.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola February 05, 2024

STORY AT-A-GLANCE
- Carnosine is a dipeptide found in meat. The highest concentrations of carnosine are found in your muscles, brain, central nervous system and gastrointestinal tract
- If you’re a vegetarian or vegan, you will have lower levels of carnosine in your muscles. This is one reason why many strict vegans who do not properly compensate for this tend to have trouble building muscle
- Carnosine binds to advanced lipoxidation endproducts (ALEs) that form from oxidized seed oils in your diet, making it a crucial aid in the detoxification of linoleic acid (LA)
- Thanks to its ability to scavenge 4-hydroxynonenal (4HNE), carnosine is also protective against obesity, diabetes, cardiovascular disease and Alzheimer’s disease, just to name a few
- The best way to optimize your carnosine level is to eat organic grass fed beef. When it comes to carnosine supplements, your best bet is liposomal versions as they have the highest bioavailability
Carnosine is a dipeptide found in meat. It’s not found in any plant foods. Dipeptide means it’s made up of two amino acids, in this case beta-alanine and histidine. The highest concentrations of carnosine are found in your muscles, brain, central nervous system1 and gastrointestinal tract,2 which gives you an indication of its potential importance.
Unfortunately, it’s also one of the top 10 most common nutrient deficiencies, especially among vegans. If you’re a vegetarian or vegan, you will have lower levels of carnosine in your muscles. This is one reason why many strict vegans who do not properly compensate for this tend to have trouble building muscle.
Carnosine also binds to advanced lipoxidation endproducts (ALEs) that form from oxidized seed oils in your diet, making it a crucial aid in the detoxification of linoleic acid (LA).
Carnosine’s Physiological Roles
Carnosine has several physiological roles and benefits. For example, it:3
| Provides athletic benefits — Approximately 99% of carnosine is found in muscle tissue where it facilitates lactic acid detoxification, improves muscle contraction and muscle relaxation and enhances endurance |
| Alleviates diabetic nephropathy by protecting podocyte and mesangial cells4 |
| Modulates energy metabolism in macrophages and microglia by restoring and/or enhancing the basal conditions |
| Has antioxidant properties and scavenges reactive oxygen species (ROS) and aldehydes created by peroxidation of fatty acid cell membranes during oxidative stress5 |
|
Regulates the activity of stem cells |
| Modulates glucose metabolism |
| Enhances the degradation and/or scavenging of nitric oxide (NO) |
| Promotes wound healing |
| Opposes glycation6 |
| Slows down the aging process by prolonging the life of cells and preserving cellular homeostasis7 |
| Regulates osmotic pressure |
| Modulates glutamate production and transport |
| Modulates brain metabolism |
| Chelates heavy metals8 |
| Acts as a pH buffer9 |
| Acts as a neurotransmitter |
| Protects olfactory receptor neurons in the elderly |
Beef, Liposomal Carnosine and Precursors Are the Best Sources
Interestingly, a June 2023 paper10 in the medical journal Pharmaceuticals reviewed the science behind carnosine with the aim of developing new delivery systems for carnosine-based drugs. As noted in this paper:
“Because of its well-demonstrated multimodal pharmacodynamic profile, which includes anti-aggregant, antioxidant, and anti-inflammatory activities, as well as its ability to modulate the energy metabolism status in immune cells, this dipeptide has been investigated in numerous experimental models of diseases, including Alzheimer’s disease, and at a clinical level.
The main limit for the therapeutic use of carnosine is related to its rapid hydrolysis … [This is the] reason why the development of new strategies, including the chemical modification of carnosine or its vehiculation into innovative drug delivery systems (DDS), aiming at increasing its bioavailability and/or at facilitating the site-specific transport to different tissues, is of utmost importance.”
Delivery systems currently in use or in development include intraperitoneal injections, intranasal sprays and oral administration of various nanoformulations. But while the drug industry is keen on figuring out how to profit from carnosine by making it into a drug, you certainly don’t need a drug to get these benefits.
Simply eating organic grass fed beef is one of the most efficient ways to raise your carnosine level.11 This is one of many reasons why cultured beef is not a viable substitute for real beef. Not only does fake beef lack carnosine but also B vitamins, retinol, long-chain omega-3 fatty acids, taurine, creatine and bioavailable forms of iron and zinc.12
Most carnosine supplements aren’t very effective either because the carnosine is rapidly broken down into its constituent amino acids by certain enzymes. Your body then reformulates those amino acids back to carnosine in your muscles.
An exception to this is liposomal carnosine, which appears to work quite well. Another alternative is to supplement with beta-alanine, which is the rate limiting amino acid in the formation of carnosine. According to a 2021 paper,13 daily intake of beta-alanine can raise the carnosine content of skeletal muscle by as much as 80%.

Download this Article Before it Disappears
Carnosine Protects Against LA-Induced Oxidative Stress
One benefit not expounded upon in the Pharmaceuticals paper is carnosine’s ability to reduce LA-induced oxidative stress. While your body will slowly eliminate stored LA over time, provided you reduce your intake, carnosine can help reduce the oxidative damage caused by LA while your body is cleaning itself out. I take liposomal carnosine every day before meals to help detoxify LA.
The omega-6 fat LA is highly susceptible to oxidation, and as the fat oxidizes it breaks down into harmful sub-components such as ALEs and oxidized LA metabolites (OXLAMs). These ALEs and OXLAMs are what cause most of the damage.
Carnosine binds to ALEs like a magnet and acts as a sacrificial sink. It’s basically a substitute target for these profoundly damaging molecules. In this way, carnosine allows your body to excrete the ALEs from your body before they damage your mitochondria, DNA or proteins. (Another molecule that protects against LA-induced damage is carbon dioxide). The illustration below shows how carnosine works in this regard.
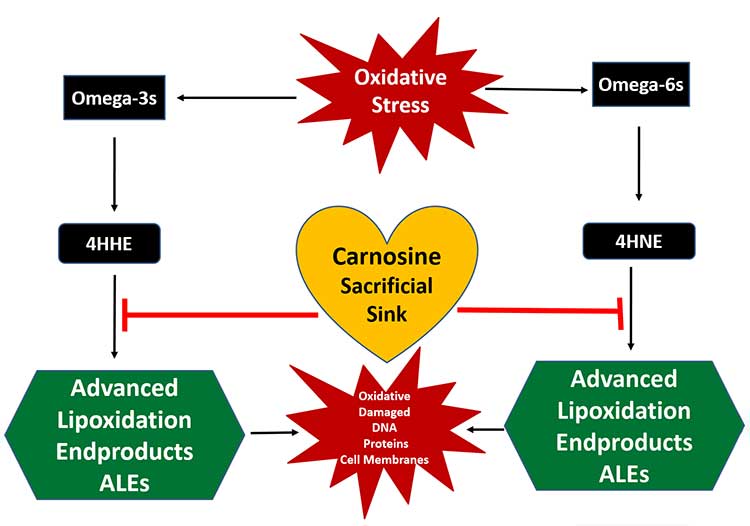
Carnosine May Be Protective Against a Wide Range of Diseases
A more detailed explanation of how carnosine protects against reactive oxygen species (ROS) and how that helps protect against oxidative stress-related pathologies is given in a 2021 paper in the journal Antioxidants:14
“A study that examined the effect of carnosine on oxidative stress in human kidney tubular epithelial (HK-2) cells indicated that carnosine decreased NADPH oxidase (NOX) 4 expression and increased total superoxide dismutase (T-SOD) activity, thus reducing the production of intracellular ROS, relieving the oxidative stress of cells, and ultimately inhibiting the mitochondrial pathway of apoptosis.
Ability of carnosine to protect against pathologies characterized by oxidative stress has been shown in a number of conditions … Carnosine changes the reactivity of superoxide anion by forming a charge-transfer complex with the superoxide radical and also by reducing the efficiency of hydroxyl radicals, creating a compound less reactive than the hydroxyl radical.
One of the mechanisms to protect organisms from oxidative stress is the chelation of transition metals, preventing them from participating in deleterious processes involving ROS … Interestingly, when comparing metals involved in free radical generation, carnosine was found to have a greater antioxidant activity coupled with copper than iron …
At physiological concentrations, carnosine directly reacts with superoxide anion similar to ascorbic acid. In physiological conditions, carnosine was found to reduce oxidative damage and to improve antioxidant activity of different antioxidative enzymes …
Experiments on aged rats showed that therapy with 250 mg/kg/carnosine per day significantly decreased oxidative stress and increased activity of antioxidative enzymes … In similar model of aged rats, carnosine increased liver vitamin E, which further demonstrates its importance in defending the organism from free radicals.
Rising data indicate that carnosine acts as a scavenger of reactive and cytotoxic carbonyl species including 4-hydroxynonenal (HNE). HNE is an aldehyde generated endogenously by lipid peroxidation of unsaturated fatty acids that act as ‘toxic second messengers,’ extending the harmful potential of free radicals.
HNE is considered an important biomarker of oxidative stress and accumulating data indicate that it may modulate signaling pathways of cell proliferation, apoptosis, and inflammation.”
How Carnosine Protects Against Alzheimer’s
As noted in the Pharmaceuticals paper,15 one of the pathologies that carnosine is protective against is Alzheimer’s disease. In my November 2021 interview with Tucker Goodrich, he explained the role of HNE, specifically, in Alzheimer’s, and why it’s so important to get rid of it.
“In heart failure, Alzheimer’s, and AMD [age-related macular degeneration], one of the things they see is an inability of the cell to produce enough energy. The mitochondria are getting damaged. HNE does that damage. It damages 24% of the proteins in the cell, primarily around energy production.
One of the ways your cells produce energy is they basically ferment glucose into pyruvate outside of the mitochondria. This is a perfectly normal part of metabolism and they produce something called pyruvate. A molecule called pyruvate dehydrogenase takes pyruvate into the mitochondria and converts it to acetyl-CoA so the mitochondria can burn it very efficiently for fuel.
Well, one of the things HNE does is it breaks pyruvate dehydrogenase, and they see this in Alzheimer’s where their cells are no longer able to produce enough energy. This is why your cells are dying in Alzheimer’s.
The beta amyloid plaques in Alzheimer’s disease are induced by HNE. There’s a great model that came out of Harvard a couple of years ago showing that.
Even the critical, the most important part of the mitochondria, complex 5, — ADP synthase — which is what takes all the energy coming from your mitochondria and turns it into ATP, which is what fuels the rest of your body — is damaged by HNE. This is a huge issue. There’s no more fundamental problem in aging and health than protein damage.”
Carnosine is the most effective scavenger of HNE, so optimizing your level can go a long way toward protecting against the HNE-induced damage that promotes Alzheimer’s.
Carnosine — A Promising Therapeutic for Obesity-Related Conditions
Elevated HNE has also been found in obese and diabetic patients,16 so there’s reason to suspect carnosine can be important in the treatment of these conditions as well. Another disease where elevated HNE plays a role is atherosclerosis. As noted in the 2021 Antioxidants paper:17
“… emerging studies have indicated that these reactive aldehydes are more than simply markers of oxidative stress.
Rather, it is suggested that these reactive species may play a significant pathogenic role in obesity-associated disorders such as insulin resistance and a carnosine analog alleviates the production or enhances the removal of reactive carbonyl species, providing promising new therapeutic compounds for cardiovascular and metabolic diseases related to obesity.”
Take Control of Your Health by Lowering Your LA Intake
As detailed in several previous articles, the evidence strongly suggests excessive LA is driving most if not all modern diseases, including heart disease and cancer. Fortunately, the solution is simple. Just lower your LA intake.
The easiest way to do this is to use an online nutritional calculator such as Cronometer to calculate your daily intake. Cronometer will tell you how much omega-6 you’re getting from your food down to the 10th of a gram, and you can assume 90% of that is LA. Anything over 10 grams is likely to cause problems. I keep my intake below 5 grams a day.
Since there’s no downside to limiting your LA, you’ll want to keep it as low as possible, which you do by avoiding high-LA foods. Keep in mind you’ll never be able to get to zero, and you wouldn’t want to do that either. You do need some LA, but since it’s found in most foods, and since you need only small amounts, there’s really no way to end up with a deficiency.
- 1, 3, 10, 15 Pharmaceuticals June 2023; 16(6): 778
- 2, 4, 5, 6, 7, 8, 9, 13, 14, 16, 17 Antioxidants July 2021; 10(7): 1037
- 11 Science Direct, Carnosine; Nutritional Supplements and Metabolic Syndrome
- 12 Animal Frontiers April 15, 2023
What’s the Best Predictor of Heart Disease?
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2024/01/18/predictors-atherosclerosis.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola January 18, 2024
STORY AT-A-GLANCE
- High total cholesterol and/or elevated low-density lipoprotein (LDL) cholesterol do not cause atherosclerosis
- Low levels of high-density lipoprotein (HDL) cholesterol are associated with both atherosclerosis and insulin resistance, and insulin resistance appears to be the foundational cause of heart disease. As such, the fasting insulin test is one of the best predictors of atherosclerosis
- Insulin resistance is primarily driven by excessive consumption of the omega-6 fat linoleic acid (LA). High LA intake is also associated with elevated levels of oxidized LDL — found in atherosclerosis plaque — further confirming this link
- One theory is that oxidized LDL protects your body from oxidative damage by sacrificing itself. If true, it may be beneficial to have higher, rather than lower, LDL levels
- The apoB test can also be helpful in assessing your atherosclerosis risk. As mentioned above, apoB is the primary carrier for LDL, and research over the past decade shows it’s an accurate predictor of cardiovascular risk when apoB is high and LDL is normal
Is high total cholesterol and/or elevated low-density lipoprotein (LDL) cholesterol indicative of elevated heart disease risk? According to Dr. Paul Saladino, the answer is no. With regard to total cholesterol, as far back as 1977, with the publication of the Framingham Study,1 no correlation between heart disease and total cholesterol could be found.
Low levels of high-density lipoprotein (HDL) cholesterol was associated with coronary heart disease, but not high LDLs or total cholesterol. However, as noted by Saladino, low HDL is also associated with insulin resistance, and he believes this is part of the confusion.
Saladino suspects that what has been blamed on LDL (atherosclerosis) is due to insulin resistance, i.e., metabolic dysfunction. Insulin resistance/metabolic dysfunction, in turn, is primarily driven by excessive consumption of the omega-6 fat linoleic acid (LA).
High LA intake also raises your levels of oxidized LDL, which are what you find in atherosclerosis plaque. In the video above, Saladino and Dr. Nadir Ali specifically discuss the role of oxidized LDL, and why it is not a direct cause of atherosclerosis, as commonly thought.
Summary of Available LDL Tests
But before I summarize Saladino’s and Ali’s discussion, let’s take a look at the available LDL-related tests, as there’s more to LDLs than the total amount.
- A regular LDL cholesterol blood test (LDL-C), which measures the total amount of LDL cholesterol in your blood
- A nuclear magnetic resonance lipoprofile (NMR lipoprofile) test, which measures the size of the LDL particles (LDL-P), which is thought to be more predictive of your cardiovascular risk, even if you have low total cholesterol2
- Oxidized LDL (oxLDL) test, which measures the level of LDLs that have been damaged by oxidation
- The apolipoprotein B (apoB) test, which measures the number of apoB particles in your blood. ApoB is a protein involved in the metabolism of lipids and the primary carrier for LDL. This test is a good predictor of cardiovascular risk, and does so far more accurately than the standard cholesterol panel
- Advanced lipid testing, which measures the amounts of large-buoyant LDLs (lbLDL) and small-dense LDLs (sdLDL), with the sdLDLs being associated with insulin resistance and heart disease3
High LDL Does Not Cause Atherosclerosis
When it comes to LDL cholesterol, the most important factor is the level of oxidized LDL, as oxLDLs are primarily what you find in atherosclerosis plaque.4 Unfortunately, most doctors will simply prescribe a statin drug or PCSK9 inhibitor if LDLs are high, to reduce the total LDL.
As noted by Ali in the short video above, this is a serious mistake. The primary question that needs to be asked is why is LDL oxidized in the first place, and how can you prevent that oxidation from taking place?
“Is the oxidized LDL a bad player?” Ali asks, “[or] is it there to protect us from oxidative injury? Rather than letting the important cells get oxidized, is the LDL sacrificing itself in protecting the body? Then, your whole paradigm changes …
LDL is not a bad player — it’s trying to protect us. What I need to figure out is how do I prevent this oxidative injury in the first place, and an argument that should surface is that, maybe I should have more LDL around so that oxidative injury can be … prevented, rather than having less LDL? These are the kinds of fundamental questions that science should be asking.”

Download this Article Before it Disappears
Oxidized LDL May Be a Protective Mechanism
Saladino agrees, saying that LDL “is probably a repository for oxidized phospholipids,” much like lipoprotein(a) (Lp(a)). He cites research showing that the more polyunsaturated fats (PUFAs) you consume — such as LA — the higher your Lp(a) and oxidized LDL.
So, your LDL may in fact have a protective rather than injurious role. It may protect you from the harmful effects of LA and other PUFAs. What this means, then, is that high oxidized LDL may be a marker of high PUFA consumption, and it’s the PUFAs, LA in particular, that are driving the atherosclerotic disease process.
The primary way to prevent atherosclerosis, then, is to radically reduce your LA intake by eliminating seed oils from your cooking, and avoiding processed foods (which are loaded with seed oils) and restaurant foods (as most are cooked in seed oils).
When it comes to measuring your oxLDL, the Boston Heart test called oxidized phospholipids on APO-B (OxPL/apoB) appears to be a better choice than the traditional oxLDL. The oxLDL tends to be inaccurate because it’s just a proxy for APO-B and the LDL number, while the OxPL/apoB test gives you a truer measure of your oxidized LDLs.
The ApoB Test
Aside from oxLDL, the apoB test can also be helpful in assessing your atherosclerosis risk. As mentioned above, apoB is the primary carrier for LDL, and research over the past decade shows it’s an accurate predictor of cardiovascular risk when apoB is high and LDL is normal. As reported by The Washington Post:5
“The standard cholesterol panel calculates the total quantity or concentration of ‘bad’ cholesterol or LDL in the blood, in milligrams per deciliter (technically, LDL-C). Because cholesterol is a fatty substance and thus not water-soluble, it must be carried around in little particles known as lipoproteins.
Testing for apoB, a protein on the outside of LDL-carrying particles, counts the number of these lipoprotein particles in the blood. In addition to LDL, it also captures other types of cholesterol such as IDL (intermediate-density lipoproteins) and VLDL (very low-density lipoproteins), which carry triglycerides.
Why is this important? As our understanding of heart disease improves, scientists are recognizing that apoB particles are more likely to become lodged in the arterial wall and cause it to thicken and eventually form atherosclerotic plaques. Thus, the total number of apoB particles matters more than the overall quantity of cholesterol that they carry.
In a majority of people, apoB and LDL-C track fairly closely, says Allan Sniderman, a professor of cardiology at McGill University in Montreal. But some people have a ‘normal’ amount of LDL-C, but a high concentration of apoB particles — a condition called ‘discordance,’ which means they are at greater risk.”
sdLDL — Another Helpful Predictor of Atherosclerosis
Measuring your sdLDL-C can also be helpful, as explained by Dr. Eric Berg, a chiropractor, in the video above. The small-dense type of LDLs are indicative of inflammation inside your arteries, which is a hallmark of atherosclerosis. As noted by Berg, potential causes of this inflammation include:
- Seed oils
- Processed foods and junk foods
- Smoking
- Low vitamin E
- High glucose levels
Unfortunately, Berg lumps high-carb diets into these risk factors and recommends a ketogenic diet to avoid elevated sdLDL, but as I’ve explained in previous articles, high glucose levels are not necessarily a sign that you’re eating too many (healthy) carbs.
In summary, when you consume significantly more than 30% fat, a metabolic switch called the Randle Cycle switches from burning glucose in your mitochondria to burning fat instead. As a result, glucose backs up into your bloodstream, thereby raising your blood sugar.
Glucose is actually a cleaner and far more efficient fuel than dietary fats, provided it’s metabolized in your mitochondria and not through glycolysis. For a refresher, refer back to “Crucial Facts About Your Metabolism” and “Important Information About Low Carb, Cortisol and Glucose.”
Atherosclerosis Is a Consequence of Metabolic Dysfunction
In the video directly above, Saladino debates LDL cholesterol with Dr. Mohammed Alo, a cardiologist and personal trainer. While Alo argues for the conventional LDL-atherosclerosis connection, Saladino highlights evidence showing that it’s not LDL per se that is the cause, but rather insulin resistance in combination with high oxLDL, both of which are caused by high LA intake.
For this reason, Saladino believes one of the best assessments of your heart disease risk is a fasting insulin test, as your insulin sensitivity is such a foundational factor of your metabolic function. The OxPL/apoB test mentioned earlier would be a good complement.
If you have high fasting insulin, you are insulin resistant and hence have some degree of metabolic dysfunction (and, of course, mitochondrial dysfunction). Ideally, you want a fasting insulin level of 3 mcg/mL or less.
Most definitely, do not go by the “normal” ranges offered by labs in this case. Many will list levels as high as 24 mcg/mL as normal, when in fact that’s a clear sign of serious insulin resistance and metabolic dysfunction.
If you’re already eating a healthy diet, exercising, and all of your metabolic parameters look good, yet you have an insulin level of 7 or 8, the core culprit may be stress, because when cortisol goes up, insulin rises with it. Cortisol release is a rescue mechanism to ensure you don’t die from low blood sugar.
To assess whether stress is at play, keep an eye on your white blood cell count. Chronically depleted white blood cells are often a sign of chronic stress and high cortisol.
You can also get an AM cortisol test after fasting for 12 to 16 hours. You don’t want to fast longer than that because, after 16 hours of fasting, cortisol will naturally start to rise. An ideal AM cortisol range is between 15 and 17. If your fasting AM cortisol is below 15, it’s a sign you’ve become stuck in a long-term stress response.
High LA Intake Promotes Atherosclerosis
So, to summarize, Saladino argues that insulin resistance is the primary root cause for atherosclerosis — not elevated LDL or total cholesterol — and the primary driver of insulin resistance is excessive LA intake from seed oils. Lowering your LA intake is the foundational strategy to embrace.6
Low levels of high-density lipoprotein (HDL) is a proxy for insulin resistance, and if you have low HDL, then LDL tracks well with cardiovascular disease. But if you have normal HDL (65 to 85 mg/dL), then you typically have good insulin sensitivity and the correlation with LDL and atherosclerosis vanishes.
The Keto Trial Match Analysis7 presented in mid-December 2023 (video below) confirms this, as they found no relationship between elevated LDL levels and arthrosclerosis plaque.
Other Tests to Assess Your Metabolic Health
In addition to the tests already mentioned, other blood tests that can help you assess your metabolic health include the following. Additional information about these and other lab tests can be found in my interviews with Dr. Nasha Winters and Dr. Bryan Walsh.
• Fasting glucose — The ideal range is between 82 and 88 milligrams per deciliter (mg/dL), based on the available literature, while nonfasting glucose should ideally be between 82 and 130 mg/dL.
• Gamma-glutamyl transferase (GGT), a powerful predictor of mortality, also should not be above 20 U/L. GGT is a liver enzyme involved in glutathione metabolism and the transport of amino acids and peptides.
Not only will the GGT test tell you if you have liver damage, it can also be used as a screening marker for excess free iron and is a great indicator of your sudden cardiac death risk.
GGT is highly interactive with iron. Excessive iron will tend to raise GGT, and when both your serum ferritin and GGT are high, you are at significantly increased risk of chronic health problems, because then you have a combination of free iron, which is highly toxic, and iron storage to keep that toxicity going.
Cysteinylglycine is liberated from glutathione via GGT. That, in the presence of iron or copper, initiates the Fenton reaction. That’s when you get massive oxidative stress.
• The Intermountain health risk score is a mortality risk score created based on the basic blood chemistry markers of tens of thousands of patients in a hospital setting, including complete blood count (CBC), sodium, potassium bicarbonate, mean platelet volume and other basics. Based on these markers, you end up with a 30-day, a one-year and a five-year mortality risk.
A risk score calculator is freely available on the Intermountain website, where you can also find more information about this score.8 Simply enter your variables and it will calculate your score for you.
• Coronary artery calcium (CAC) scan — This test provides images of your coronary arteries. Existing calcium deposits, an early sign of coronary artery disease, will show up on these images, and can therefore reveal your risk of heart disease before other warning signs become apparent.9
For even more information about cholesterol, and why high cholesterol and/or high LDL are not risk factors for heart disease, check out the video below by Dr. Ken Berry.
- 1 American Journal of Medicine May 1977; 62(5): 707-714
- 2 Front. Nuci. Med July 13, 2022; 2
- 3 Health Matters LDL Size
- 4 Mediators of Inflammation 2013; 2013: 714653
- 5 Washington Post January 8, 2024
- 6 Chem Phys Lipids January 1998; 91(1): 1-11
- 7 Citizens Science Foundation December 13, 2023
- 8 Intermountain Risk Scores
- 9 Hopkins Medicine CAC Test
How to Produce the Healthiest Foods Imaginable
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2023/12/31/food-production.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola December 31, 2023
STORY AT-A-GLANCE
- Low-carb/high-fat diets ultimately backfire because they inhibit glucose metabolism, which is the most efficient form of energy production in the mitochondria; they also impair thyroid function
- One of the reasons why ketogenic and carnivore diets are usually helpful for a time is because, if implemented properly, you’re radically reducing your intake of omega-6 fats, linoleic acid (LA) in particular, which is one of the primary drivers of ill health
- LA is a primary driver of disease, in large part due to its detrimental effect on mitochondrial function and, hence, energy production
- Your body has a certain amount of energy and a number of biological processes that it can turn on or turn off with that energy pool. The more energy you have available, the more functions your body can turn on. When your energy production is lower than required to maintain all functions, your body must downregulate certain functions, which ultimately results in problems
- One of the easiest ways to assess how much energy your body is producing is to take your body temperature. Take your temperature 30 to 40 minutes after breakfast and midday. You want to see a rise in temperature
The interview above features Ashley Armstrong, who’s an expert in two areas. One is producing some of the healthiest food in the United States, and the second is understanding how your body uses it and how to select the right types of food to optimize your biology, based on the late biologist and thyroid expert, Ray Peat’s, principles of bioenergetic medicine. She also is a certified personal trainer with a Ph.D., MS and BS in engineering.
Like many others who are trying to improve their health, Armstrong tried low-carb diets, fasting, keto and even carnivore diets in the past. But while these all led to improvements initially, they didn’t eliminate them, which ultimately led her to investigate Peat’s principles.
“Ray Peat, he honestly saved my life and I owe so much to that man,” she says. “I’m forever grateful for him. The biggest wake-up for me was measuring my body temperature. I was on a carnivore diet and measured my body temperature — it was 96.5 degrees Fahrenheit.
I was like, wow, no wonder my hair is thinning. No wonder my complexion is so pale. No wonder I’m not sleeping through the night. There was just a number of red flags. That body temperature measurement just woke me up. It’s what I needed to [realize] I’m not thriving, I’m just surviving.
I’ve been implementing Dr. Peat’s principles for over three years now. I have more energy in life than I think I’ve ever had, even as a teenager. And it’s just amazing to see how being not restricted with your food, just being strategic with macros, types of food, how powerful that can be for your energy production.”
The Problem With Low-Carb and Keto
As I’ve detailed in previous articles over the past year, low-carb/high-fat diets ultimately backfire because they inhibit glucose metabolism, which is the most efficient form of energy production in your mitochondria; they also impair thyroid function. Your thyroid is crucial for energy production, and if your thyroid doesn’t work, you’re down the creek without a paddle.
One of the reasons for this is because ketogenic diets increase the stress hormones — cortisol, glucagon and adrenaline. On the other hand, one of the reasons why ketogenic and carnivore diets are usually helpful for a time is because, if implemented properly, you’re radically reducing your intake of omega-6 fats, linoleic acid (LA) in particular, which is one of the primary drivers of ill health.
Energy Production Is Key for Overall Health
As explained by Armstrong, the best way to understand the bioenergetic principle is to think of your body as a system. It has a certain amount of energy, and a number of biological processes that it can turn on or turn off with that energy source.
The greater your energy pool, the more functions your body can turn on. When your energy production is lower than required to maintain all functions, your body must downregulate certain functions, which ultimately results in problems. The human body is designed to promote survival, so it’s going to prioritize things like your heart rate.
Functions that aren’t necessarily vital for survival in the immediate moment, like sex hormone production, reproductive function, digestion, sleep and high cognitive thinking, get downregulated first. When you increase energy production, however, your body can then expend energy on those functions and bring them “back online.”

Download this Article Before it Disappears
Using Body and Pulse Measurements as Guides
As explained by Armstrong, one of the easiest ways to assess how much energy your body is producing is to take your body temperature.
“High stress hormones can keep your waking body temperature elevated,” she says, “so you’ve got to do your waking temperature 30 to 40 minutes after breakfast, and then I like to do midday. You want to see that temperature rise.
For many who are on low-carb or who are living on stress hormones, they’re going to have potentially high waking body temperature, but after breakfast, that temperature may drop. That’s because the food you’re consuming is lowering your stress hormones and your actual body temperature is then better exposed.
So we want to see that body temperature rise. And I love how both of us are so passionate about linoleic acid. As human linoleic acid consumption has gone up, human body temperature has gone down. So, the types of fats that we are consuming in our diet is impacting energy production in a negative way.
It’s shown with obesity rates out the roof. It’s shown with the decline in our body temperature. It’s shown with the decline in our healthy life expectancy, which is bizarre as a First-World country. There are just so many profound effects.
But when we just think of it as energy production — the more energy we can give our body to be able to perform functions, the better it’s going to function. I asked this question to someone who is really adamant about fasting. I said, ‘If you’ve got two bodies, one body that’s fasted and the other body that is fed nourishing food, which body is going to thrive and function better?’
It’s obvious. If you add a third person fed more of a standard American diet, of course maybe fasting is going to make you feel better, but you can elevate yourself a step above. You don’t have to rely on fasting to increase energy production. Your body is not going to increase energy when you’re not [putting] energy in.”
Indeed, when it comes to fasting, one of the primary benefits is that it lowers the fuel for gram-negative bacteria that produce endotoxin in your gut. Low-carb does this as well. Endotoxin, estrogen, LA and stress hormones will all decrease your mitochondrial function, mediated in big part by your thyroid function. Those are the big things that need to be reduced to enhance your mitochondrial function and energy production within the mitochondria.
How LA Harms Your Energy Production
As mentioned, LA is a primary driver of disease, in large part due to its detrimental effect on mitochondrial function and, hence, energy production. Your body can use both fat and glucose for energy. Muscle, in particular, will use fat for fuel, as will your heart. So, fat is not bad, but it’s important to realize that different fats affect your body in different ways, so it’s crucial to get the right fats. Armstrong explains:
“The different types of fatty acid molecules have drastically different structures and those impact the internal environment inside of us. They impact how your body is producing energy. The more saturated we can become, the better our internal environment is going to be.
When someone goes low-carb, maybe they reduce the amount of packaged food that they’re eating that contains a ton of vegetable oil and linoleic acid, and so potentially they’re resaturating some of their tissues.
But when you learn about what livestock are being fed these days, then you realize that a high animal fat diet can still contain quite a bit of PUFAs [polyunsaturated fats] and linoleic acid, depending on what those animals ate. So, think it’s important to consider the amount of each macronutrient that you’re intaking because that can have profound impacts on your energy production.
Saturating your tissues is going to take you to the next level, but adding in appropriate levels of carbohydrates is going to allow you to take your consciousness and energy production level to the next level [beyond that].”
The types of carbs you eat matter, however. I’m convinced the ideal carbohydrate is fresh, ripe fruit. Ripe is the key here. Of course, some fruits are better than others. Watermelon, for example, is among the best. Watermelon with feta cheese and a little mint on top makes for a delicious snack.
Aside from containing a lot of water, watermelon also contains a substance called citrulline, which converts into arginine, a precursor for nitric oxide (NO). NO is important to your body, but the caveat is that it needs to come from real food. Drugs like Cialis or Viagra, which act by increasing NO, will accelerate your path toward premature death. Artificial citrulline and other synthetic amino acids that raise NO are also best avoided.
“In Michigan, I rely a lot on frozen fruit,” Armstrong says. “In the summertime I’ll go to strawberry fields and pick strawberries when fresh and then freeze a ton of them. Same thing with blueberries and peaches. And then I rely on a lot of apples in the winter because apples are abundant around here and can be stored.”
Juices also have their place. Cold-pressed, pulp-free orange juice, for example, is a good choice. The reason you want pulp-free is because if you’re like most people, you have gram-negative, endotoxin bacteria in your gut that will thrive on the pulp, hence increasing endotoxin production.
So, if you have an unhealthy microbiome, pulp-free orange juice is a great carb that will gently and safely allow you to enter the higher carb world. As your microbiome improves, then you can transition to whole fruits and berries, which is, I believe, far superior to juices.
How to Produce the Best Eggs
Segueing into the topic of food production, Armstrong’s farm produces some of the highest quality eggs I’ve ever come across, and the feed recipe I use for my own chickens came from her. But I recently discovered something that could make them even better, and that is to allow the chickens to scratch for their own food.
Their ideal food is insects fresh from the ground, and while I previously thought chickens couldn’t get enough food this way, meaning you had to give them something, that may actually not be true.
Unfortunately, in places where the ground freezes, chickens will not be able to sustain themselves on insects, and you definitely do NOT want to feed your chickens dehydrated bugs. Why? Because the bugs are raised on corn and soy, making them very high in LA.
But in places like South Florida, for example, you can easily produce top-notch eggs, quality-wise, by allowing your chickens to peck for insects, without giving them any supplemental feed. Armstrong is also making plans to let her chickens forage for bugs year-round:
“I think that would be the ideal condition, and I have an image in my head of what I want to bring our farm to in the future — a greenhouse where we’ve got fodder growing on the ground and a worm farm … so [the chickens] will get abundant bugs in the winter. That’s what I want to move towards, but that requires a lot of financial investment. So we’ll get there one day.”
The Feed Has Dramatic Impacts on Animal Foods
The feed Armstrong developed, which I’ve been using as well, results in eggs that have about 75% less LA than conventional eggs. When it comes to conventional eggs, the LA is really the only problem. When the chickens are fed an ideal diet, the yolk in the egg is one of the best, most nutritious foods imaginable. The only thing that comes close is organ meat.
Egg yolks are the ultimate food; the problem is 99.99% of the eggs produced in this country are not that good. I don’t care if they say free range, grass fed, organic, it doesn’t matter. They’re terrible because they have four times more LA than they should. As noted by Armstrong:
“It’s important to consider organic soybeans have the same amount of linoleic acid as non-organic soybeans. Whether it’s grown conventionally, organically does not change the fatty acid composition of soybeans. You don’t want to be eating eggs from chickens fed a bunch of soy vegetable oil and other high omega-6 PUFA foods.”
According to Armstrong, the feed of the chickens may even determine the eggs’ allergenicity. In other words, if you’re allergic to eggs, you could potentially be able to eat the eggs from correctly-fed chickens.
“What is soy high in? Phytoestrogens that can be very problematic for some people. If a chicken is eating phytoestrogens that can be problematic for humans, those get passed through into the eggs. We have a number of customers that cannot eat any other eggs, but they’re totally fine with our eggs. And it’s because of the diet of the chicken.
So if you have allergic reactions or problems with eggs, try a different source where they’re not fed soy. Some people can be allergic to corn as well, and that allergenicity can pass through the egg as well. But it seems like soy is the biggest culprit.
But be careful of many corn and soy-free feeds, because those are high-PUFA ingredients like sunflower, flax, fish oil, vegetable oil and safflower oil. And so, just be really careful of your source, and ask what the chickens are eating. But yes, allergenicity of eggs I think really depends on what the chicken eats.”
LA-Rich Animal Feed Is Now Impacting Human Energy Production and Health
All of that said, it’s still crucial to ensure your chickens have enough food, be it fresh insects or a carefully planned feed that is low in LA and high in healthy saturated fats and other nutrients.
“Your chicken is not going to thrive if it’s underfed,” Armstrong says. “Your chicken is not going to thrive if it doesn’t have food. I am trying to boost the metabolic rate of our chickens as high as possible. Just like us, chickens are monogastric single stomach animals, the types of fat that they are fed, the types of fat that we are fed impacts the types of fat inside of us.
This is a little bit different for ruminant animals — cows, goats — but for monogastric chickens, pigs, their diet is very important. And this is why I am so passionate about it, because we have been lied to and convinced that saturated fat is bad for us.
So, you’ve seen a huge push for PUFAs in our diet. This is going beyond just human dietary choices. This is impacting our livestock food. And this is having profound impacts on not only livestock health, but also the food that we’re consuming …
Even in the dairy industry, they’re creating things called rumen-protected fats. They are PUFAs that in a typical rumen digestion system can go through the process called hydrogenation, which turns the PUFA into saturated fat.
They are designing rumen-protected fats so that the PUFA is passed through the rumen. The PUFA content of milk is increasing. That means any dairy fat — butter, cream, whole milk. The PUFA content of beef fat is increasing. And this is by design … Lard and chicken fat from conventional animals has the same amount of PUFA as canola oil.
This is profound. We have changed the types of fat inside of us. I think the linoleic acid content of humans has increased 136%. That is changing how our body is making energy inside of us. The types of fat we consume day-to-day have a long life inside of us — 600 days. So, the types of fat we’re consuming day to day impacts our energy production for years to come.
It’s unfortunate because this is just the reality for a lot of people, and that’s why I’m so passionate about it. Our food system is designed in a way that is not setting us up for success. That’s why I want to try to change it by going back to how our food was produced 100 years ago, where there was appropriate amounts of PUFAs in foods, small amounts, and saturated fat was the predominant fat source for both livestock and humans.”
High PUFA Diets Shut Down Your Metabolism
As explained by Armstrong, in nature, animals increase their PUFA consumption up to a certain amount to initiate torpor, which means their metabolism is so downregulated that they can survive the winter without eating. Think about that. Can you function optimally if your diet is one meant for hibernation? In that state, you have to eat fewer and fewer calories to avoid weight gain, which results in undernourishment and poor energy production.
“I try to keep my PUFA consumption as low as possible,” Armstrong says. “You can easily track that in Cronometer and see what your total PUFA, total linoleic acid content is per day. If you have four conventional eggs, you’re already at about 5 grams of linoleic acid in a day. And I would want people to be lower than that. All foods contain some amount of linoleic acid, so even milk is going to have a little bit.”
There’s no question that LA is NOT an essential fat, even though it’s categorized as such. It’s not essential because nearly all foods contain it. It’s virtually impossible to become deficient in LA if you eat food, regardless of what that food is.
Another fat that likely IS essential, but isn’t widely recognized as such, is the odd-chain saturated fats (OCFAs) found primarily in dairy. You can learn more about this in “The Amazing Benefits of Dairy Fat.” There’s also evidence suggesting that if you don’t get enough OCFAs in your diet, then high saturated fat intake might become problematic.
So, you need these odd-chain saturated fats. That’s why you need butter. You need milk. These are essential. Your optimized biology and health is dependent on these foods, because, again, the OCFAs help increase your body’s energy pool. They boost energy production, which will improve how your entire body functions.
In the interview we also discuss how dairy improves the health benefits of eggs, as the calcium in the dairy reduces the conversion of tryptophan in the egg white into serotonin. Serotonin is another compound you simply do not want too much of.
You also want to make sure you’re having enough carbohydrates with that meal. Carbohydrate oxidation produces 50% more carbon dioxide (CO2), so simply having carbs with your eggs will raise your CO2 level, which is very important for health.
“So, for breakfast, have eggs, milk, some honey or maple syrup and fruit. Boom, there you go. You’re drastically reducing the conversion of tryptophan to serotonin and it’s a simple meal,” Armstrong says.
More Information
We discuss a lot more in this interview than what I’ve covered here, so for more fascinating details, be sure to listen to the whole interview. For example, we discuss the pros and cons of egg whites, and why most cheese sold in the U.S. is less than healthy, as many cheese producers are using a microbial rennet made by Pfizer that is derived from mold that eats genetically modified corn and soy.
We also discuss various ideas for improving the feed of chickens, and how to maintain maximum egg production in the winter with incandescent lights and red light therapy.
If you want to purchase eggs from Armstrong’s farm, Angel Acres Egg Co., visit angel-acresfarm.com. She’s also started a new private member food system that offers milk, cheese, low-PUFA pork and low-PUFA chicken, called Nourish Cooperative. Both will ship farm-fresh food right to your door.