ATP
now browsing by category
Glucose — The Ideal Fuel for Your Cells
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2025/02/19/glucose-mitochondria.aspx
Analysis by Dr. Joseph Mercola February 19, 2025

STORY AT-A-GLANCE
- Glucose is the most efficient fuel source for cellular energy production, generating more ATP (energy) per oxygen molecule compared to fats, making it especially valuable during low-oxygen conditions like intense exercise
- Unlike fats, glucose can produce energy even without oxygen through a process called glycolysis. This serves as a crucial backup energy system during high-intensity activities when oxygen is limited
- Glucose serves as a versatile building block in the body, participating in essential pathways like gluconeogenesis (creating new glucose from proteins) and the pentose phosphate pathway, which produces crucial components for DNA and RNA
- Your body stores excess glucose as glycogen in the liver and muscles, creating readily available energy reserves that can be quickly accessed between meals or during exercise
- While fats are excellent for long-term energy storage, they require more oxygen to break down and can produce more harmful reactive oxygen species (ROS) compared to glucose during energy production
Cells contain specialized organelles called mitochondria that are responsible for cellular energy production. Mitochondria generate adenosine triphosphate (ATP), which functions as the primary energy molecule used by cells to power essential biological processes,1 including muscle contraction and nerve signal transmission.
Mitochondria can metabolize both glucose and fats to produce ATP through a series of biochemical reactions. However, research indicates that glucose serves as the most efficient and versatile substrate for mitochondrial ATP synthesis, as the metabolic pathways involving glucose yield more ATP per molecule of oxygen consumed compared to fatty acid oxidation.2,3,4,5,6,7,8,9,10
To understand why glucose shines so brightly as a fuel source, it helps to zoom in on a few key concepts: how glucose delivers energy, why its metabolic “exhaust” tends to be less stressful on your cell’s machinery, and how relying on other fuels like fats can introduce imbalances and potential harm. By the end, you’ll see that glucose provides a balanced and high-yield form of energy production, effectively preventing certain risks that come with over-reliance on other fuels.
Why Glucose Is the Best Fuel
First, glucose enters your cell and gets broken down into smaller pieces outside the mitochondria, in the main part of the cell. This is like preparing the ingredients before cooking a big meal — you’re chopping up the vegetables before you start cooking. This step produces a little bit of energy, but not much. It’s like a small spark before the fire really starts.
These smaller pieces, the chopped-up glucose, then enter your mitochondria, where the real action begins. They go through a cycle of changes, a bit like a series of dance steps or a recipe with multiple steps. This is where the main process of breaking down glucose happens. This process creates special “energy carriers” that are like delivery trucks ready to transport energy to where it’s needed in your cell.
Finally, these energy carriers go to a special area in your mitochondria, which you can imagine as a power plant’s control room or a generator. They deliver their “energy cargo” through a chain of events like an assembly line in a factory. As the energy cargo moves along this line, it creates a flow of power that spins a tiny “turbine” or a water wheel. This spinning generates the main energy currency of your cell, ATP.
This whole process can create significant energy from one molecule of glucose, much like getting a lot of mileage from a full tank of gas. Glucose is a such a great fuel because it creates more of these special “energy carriers” early on, compared to other fuels like fats. These carriers are very efficient at delivering their energy to create ATP. This makes glucose the star of the show when it comes to powering your cells, ensuring they have the energy they need to function properly.
Why Fat Isn’t Always the Best — The Reductive Stress Angle
We often hear about fats as a super fuel because they hold large amounts of stored energy. Imagine a huge barrel of oil that can power a city for days. It sounds great — until you discover that extracting energy from that oil might require more oxygen, produce more damaging fumes, or strain the city’s power lines with surges and brownouts.
In a similar way, while fats can give your mitochondria plenty of stored energy, they are not always the easiest or safest fuel to burn.11,12,13,14,15,16,17
Here’s the main difference: fats and glucose produce different types of “energy carriers” during their breakdown. These carriers transport electrons, which are tiny particles with a negative charge, to your cell’s mitochondria. One type of carrier is called FADH2, and the other is called NADH. Think of FADH2 and NADH as different models of delivery trucks, each designed to carry electrons but using slightly different routes and having slightly different efficiencies.
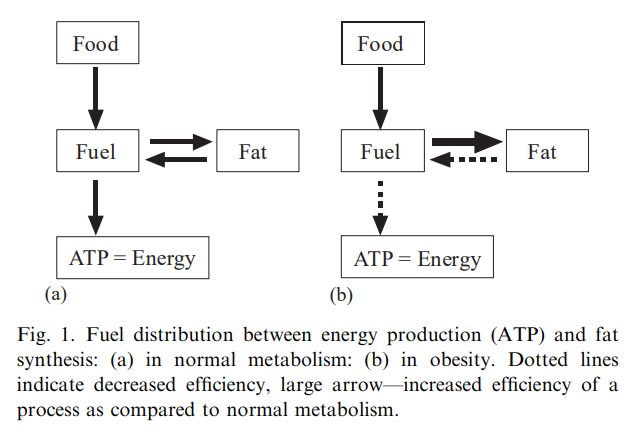
When fats are broken down, they tend to create more FADH2 than NADH. The problem is that FADH2 enters the energy production process, called the electron transport chain (ETC), at a later stage (Complex II).18 It’s like a delivery truck that takes a longer, less efficient route. Because of this later entry point, fewer ATPs are made.
Another issue with relying heavily on fats for energy is the risk of something called reductive stress. Reductive stress happens when there are too many electrons (negative charges) flooding your system. Imagine an electrical circuit in your house: if you plug in too many high-powered appliances at once and overload the circuit, you risk sparks, short circuits, or even a power outage.
Similarly, in your cell, this overload of electrons leads to the formation of harmful molecules called reactive oxygen species (ROS).19 These ROS are like the “sparks” in our overloaded circuit analogy. They can damage important parts of the cell, such as proteins and DNA, similar to how sparks can damage your home’s wiring.20
A surplus of negative charge from the electrons that are not handled in an organized manner is called reductive stress. This is a relatively recent concept and was not discovered until 1989.
On the other hand, when glucose is broken down, it produces mostly NADH. NADH is like a more efficient delivery truck that takes a direct route, entering the electron transport chain at the very beginning (Complex I).21 This allows for more ATP production because the electrons have a longer runway as they enter the electron transport chain much earlier. This also tends to produce fewer harmful ROS “sparks.”
Furthermore, glucose has another advantage: it can still be used to produce some energy even when oxygen levels are low, a process called glycolysis.22,23 Fats can’t do this efficiently. So, if you’re doing intense exercise like sprinting or are in an environment with less oxygen, your cells can still partially rely on glucose to generate ATP,24 even if it’s not as much as when oxygen is abundant.
It’s like having a backup generator that can still provide some power even if the main power source is low.

Save This Article for Later – Get the PDF Now
Glucose in Day-to-Day Life — More Than Just Energy
Glucose is much more than just a source of immediate energy for your cells; it plays a central role in a wide variety of other essential processes that keep your body functioning smoothly. You can think of glucose as a central hub connected to many different metabolic “side roads” that allow your cells to store energy, build necessary molecules, and recycle components as needed.25,26 These pathways are important for maintaining the body’s overall metabolic balance.
One of these important pathways is called gluconeogenesis.27 This Latin term, “gluconeogenesis,” simply means that your body can create new glucose from existing fuel sources in your body, primarily from protein.
While this ability to make glucose from non-carbohydrate sources is a magnificent backup system, ensuring the body has a supply of this essential fuel during times of need, relying on it as a primary source of energy can have powerfully destructive consequences, such as muscle wasting.
Another crucial pathway is glycogenesis. This process is like the body’s way of storing extra glucose for later use. When there’s an abundance of glucose, such as after a meal, the body converts it into a storage form called glycogen, primarily in the liver and muscles.28 Glycogen is like a quick-release fuel reserve that can be rapidly broken down back into glucose when energy demands increase, such as during exercise or between meals.
Then there’s the pentose phosphate pathway. This pathway is less about generating ATP (the cell’s main energy currency) and more about producing other important molecules, namely NADPH and ribose-5-phosphate.29 NADPH is a crucial molecule that acts as a reducing agent, meaning it donates electrons when your body needs them to run other metabolic processes.
The pentose phosphate pathway is a special process in your cells that doesn’t focus on making energy (ATP) directly, but instead creates other essential building blocks. You can think of it as a side road in your cell’s metabolic network, branching off from the main energy production route.
One of the key products of this pathway is NADPH. As mentioned before, NADPH is a molecule that acts like a delivery truck for electrons. It carries electrons and donates them in various cellular reactions. Why is this important? Well, many reactions in your body require electrons to proceed, and NADPH provides them.
The other important product of the pentose phosphate pathway is ribose-5-phosphate. This is a type of sugar molecule, but not the kind you eat. Ribose-5-phosphate is an essential component for building the genetic material of your cells, specifically DNA and RNA.30
DNA carries the instructions for building and operating your entire body, while RNA helps carry out those instructions. So, ribose-5-phosphate is essential for creating new cells, repairing damaged tissues, and generally keeping your body functioning properly. It is a five-carbon sugar that is essential for building the framework of DNA and RNA.
Glucose Is an Essential Building Block
Because glucose is involved in all these interconnected pathways, including gluconeogenesis, glycogenesis, and the pentose phosphate pathway, it acts as a truly versatile building block that can be used in many ways. Unlike fat, which is primarily an energy storage molecule, glucose can be readily converted into other essential molecules, used for immediate energy, or stored for later use.
It’s not just a quick source of energy; it’s also a fundamental component that helps maintain your body’s overall metabolic balance, ensuring that your cells have all the resources they need to function, grow, and adapt to changing conditions.
Only glucose can perform the miracle of being created from non-carb sources, a process called gluconeogenesis, highlighting its unique and indispensable role in the body. It’s like a universal puzzle piece that can fit into many different slots, making it an essential player in the complex network of cellular processes.
This ability to be interconverted between different forms and to participate in diverse pathways makes glucose, and not fat, the essential metabolic player it is. It’s not merely a short-term energy fix; it helps maintain your body’s broader metabolic balance, something that fat cannot achieve.
What Happens When There’s Too Much Glucose?
Glucose may be the ideal fuel, but your body prefer a “just right” approach. If glucose levels become chronically high — whether because of poor dietary habits, stress, or insufficient insulin action — your cells will suffer. One major problem is glycation, where glucose sticks to proteins, forming advanced glycation end products (AGEs) that can accumulate like sticky residue in a machine.31,32
Over time, these AGEs can degrade the function of tissues, triggering inflammation or stiffening blood vessels.
Excess sugar in your bloodstream also prompts more ROS production. While some ROS are part of normal cell signaling, too many damage mitochondria themselves, leading to even less efficient energy production.33,34
In that sense, flooding your system with glucose is like sending too many packages through a conveyor belt: at first, everything hums along, but eventually, congestion and accidents happen. Balancing glucose is key to letting the mitochondria work optimally, free from the chaos that extremes in sugar can create.
Type 2 diabetes offers a prime illustration of how essential glucose balance is. In diabetes, cells no longer respond properly to insulin, which normally helps cells take in glucose. Despite high blood sugar, many cells starve for energy, and mitochondria become less effective at generating ATP.35
Over time, tissues and organs suffer: small blood vessels deteriorate, nerves may be damaged, and the risk of heart disease climbs. This meltdown highlights how simply flooding your bloodstream with sugar isn’t enough; glucose must reach your mitochondria in a controlled way.
The heart and brain, known for their heavy energy demands, also show how valuable glucose can be. During a heart stress event or intense mental task, glucose provides far quicker energy per oxygen molecule than fat.36 This is particularly important if oxygen supply is in short supply — like a clogged artery in the heart or a momentary oxygen drop in the brain.
Summary — Why Glucose Earns the Title ‘Ideal Fuel’
So, in summary, glucose provides your cells with a highly efficient and adaptable way to generate energy and perform other essential functions. One of its key advantages is that it offers a high ATP yield per oxygen molecule used. ATP is the primary energy currency of your cells, like the electricity that powers your house.
When your cells break down glucose, they produce more ATP for every molecule of oxygen they consume compared to when they break down fat. This is especially important for tissues that sometimes experience low oxygen levels, such as your muscles during intense exercise. Think of it as getting more energy output for the same amount of input — glucose is simply a more efficient fuel in this regard.
Furthermore, glucose has the remarkable ability to generate energy even without oxygen. During short bursts of high-intensity activity, like sprinting, your muscles might need energy faster than your body can deliver oxygen. In these situations, your muscle cells can still produce ATP through a process called glycolysis, which breaks down glucose without needing oxygen.
Fat breakdown, on the other hand, always requires oxygen, so it can’t provide this emergency energy boost.37 It’s like having a backup generator that can kick in when the main power source is unavailable. Glycolysis is unique in that it does not require oxygen to proceed.
So, to reiterate, glucose isn’t just about providing energy; it’s also incredibly flexible in how it can be used by the body. It participates in various metabolic pathways, allowing for the creation of many necessary compounds.
For instance, glucose metabolism helps produce NADPH, a molecule that acts like a delivery truck for electrons, which are needed for various cellular processes, including building and maintaining your body’s antioxidant defenses. These defenses protect your cells from damage caused by ROS. In addition, your body can store extra glucose as glycogen, primarily in your liver and muscles.38,39
Glycogen is like a reserve tank of fuel that can be quickly tapped into when your body needs a rapid energy supply, such as between meals or during exercise.
Why Glucose Beats Fat for Everyday Energy Needs
Finally, when glucose is broken down, it mainly produces NADH, another type of electron carrier. NADH enters the cellular energy production machinery to the electron transport chain in your mitochondria, at the very beginning (Complex I), which helps maintain a smooth and efficient “electron flow,” like a well-regulated electrical current.
Relying too much on fat for energy can lead to an overproduction of FADH2, a different type of electron carrier that enters the machinery later (Complex II). This disrupts the electron flow, creating reductive stress.40,41 Reductive stress is like an overloaded electrical circuit, where too much negative charge builds up, increasing the risk of producing those harmful “sparks” called ROS, which can damage your cells. Maintaining a balance of electrons is essential.
Some people might point out that humans evolved to store a lot of fat for a reason, and that’s true. Fat is an excellent long-term energy reservoir, crucial for surviving periods of famine or prolonged food scarcity. However, in everyday situations, especially those involving physical activity or fluctuating oxygen levels, glucose offers a far more versatile and efficient way to meet your body’s energy and metabolic needs. It can be used to produce energy quickly even without oxygen.
It can generate important molecules needed to maintain your cellular health and regulate electron flow to prevent damage, and also be used to create other molecules in essential processes. So, while fat is a vital energy reserve for long-term survival, glucose is the preferred fuel for optimal performance in most day-to-day activities and plays a much broader role in supporting overall metabolic health.
Balancing Glucose for Optimal Mitochondrial Function
From top to bottom, glucose shows itself to be the “special sauce” for keeping your mitochondria humming along at peak capacity. Its biochemical pathways are poised to spin out large amounts of ATP, carefully managing the electron flow to avoid redox chaos.
While glucose is clearly a vital fuel for the body, it’s important to understand that it’s not a free pass to consume excessive amounts of sugar. Your body thrives on balance, and just like with anything else, too much glucose is detrimental. Excess glucose leads to harmful processes like glycation, where sugar molecules bind to proteins and impair their function, and oxidative stress, an imbalance between free radicals and antioxidants that can damage cells.42,43,44
Chronically elevated blood sugar levels can contribute to serious health problems, including Type 2 diabetes, heart disease, and even cognitive decline.45,46,47,48,49,50,51
One of the marvels of your biology is that maintaining moderate glucose levels, coupled with good insulin sensitivity, allows your cells to enjoy the best of both worlds. Insulin sensitivity refers to how effectively your cells respond to insulin, a hormone that helps regulate blood sugar. When insulin sensitivity is high, your cells can efficiently take up glucose from the bloodstream and use it for energy or store it for later use.
This allows cells to reap the benefits of glucose as a fuel source while avoiding the negative consequences of both excessive sugar intake and an overreliance on fat for energy.
A crucial piece of this complex equation is understanding your current state of metabolic health. Factors like your exposure to mitochondrial poisons and gut health status play major roles in determining how your body processes glucose and what your optimal carb sources are. In short, your individual metabolic health determines which carbohydrates will support your health and which will be detrimental — and this can change over time.
Future articles will delve deeper into the fascinating interplay between metabolic health, individual variations, and personalized approaches to optimizing glucose metabolism.
- 1, 12 Khan Academy, Cellular Respiration
- 2, 11, 18, 22, 24 Osmosis, Cellular Respiration — What Is It, Its Purpose, and More
- 3, 35, 45 Physiology, Glucose Metabolism. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; January 2025
- 4, 31, 42 Wikipedia, Glucose
- 5 Lift Glucose, August 22, 2024
- 6 Libre Texts Biology, 5.9: Cellular Respiration
- 7 Vedantu, Do mitochondria use glucose?
- 8, 10, 13, 25, 38 Biochemistry, Aerobic Glycolysis. [Updated 2023 Apr 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; January 2025
- 9 Cayman Chemical, April 22, 2019
- 14, 21, 23, 27 Eur J Clin Nutr 53 (Suppl 1), s107–s111 (1999)
- 15, 20, 43 PLoS One. 2023 Sep 21;18(9):e0289475
- 16, 33 J Inflamm Res. 2025;18:115-126
- 17, 32, 34, 44 Diabetology 2022, 3(4), 583-595
- 19 Eurasian J Med. 2018 Oct;50(3):193–201
- 26, 28, 29, 30, 39 AMSBIO, Post Glycolytic Pathways
- 36 Compr Physiol. 6(1): 331–351
- 37 Clin Sci (Lond). 1980 Dec;59(6):469-78
- 40 Antioxid Redox Signal. 2020 May 14;32(18):1330–1347
- 41 Antioxid Redox Signal. 2020 Jun 26
- 46 Int. J. Mol. Sci. 2023, 24(5), 4394
- 47 Circulation Research February 29, 2008; 102(4)
- 48 J Diabetes Investig. 2010 Jul 6;1(5):161–169
- 49 Metabolites. 2022 Jul 29;12(8):712
- 50 Medical News Today, September 23, 2021
- 51 Cancer Research UK, August 16, 2023
The Engine of Life — Understanding Mitochondrial Energy Production
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2025/02/05/mitochondrial-energy-production.aspx
Analysis by Dr. Joseph Mercola February 05, 2025

STORY AT-A-GLANCE
- Just like a car can’t run without gas, your body can’t function without cellular energy. Yet doctors often treat symptoms while missing the real problem — an energy deficit at a cellular level
- Your cells have tiny powerhouses called mitochondria that produce 90% of the body’s energy through oxidative phosphorylation; the mitochondria also regulate calcium levels, cell death and metabolic processes
- Adenosine triphosphate (ATP) is your body’s energy currency, made up of sugar, nitrogen and three phosphate groups. Your body makes its weight in ATP daily to power everything from muscle movements to brain signals
- Mitochondrial dysfunction and resulting energy deficits are linked to numerous diseases, including diabetes, cancer, neurodegenerative conditions, autoimmune disorders and cardiovascular disease
- Instead of just treating symptoms, modern medicine needs to focus on restoring cellular energy production. This will revolutionize how we manage diseases by tapping into the body’s natural healing abilities
A diseased body is like a car that has run out of gas. The engine might be intact, the tires perfectly inflated and the body free of dents, but without fuel, the car won’t move. Similarly, the human body cannot function without energy.
Unfortunately, modern medicine often resembles a mechanic diligently fixing flat tires or replacing spark plugs in a car that’s simply out of fuel. No amount of tinkering will get it moving if the real issue is not addressed.
This is precisely what happens when surface-level symptoms are treated without addressing the root cause of diseases — a profound energy deficit at the cellular level. Every process in your body depends on cellular energy produced by the mitochondria. When energy levels are sufficient, your body can repair and regenerate, even if the damage is severe. But when energy production falters, your body stalls, healing slows and chronic illnesses take hold.
The current approach to medicine fails to acknowledge this fundamental truth. By focusing on managing symptoms, it offers temporary fixes rather than lasting solutions. True healing requires shifting the focus to restoring cellular energy production — the very foundation of health. Only by optimizing this energy system will you be able to unlock your body’s innate ability to heal and thrive.
Understanding Mitochondria — The Powerhouse of Cells
Inside nearly every cell in your body are structures that sustain life as you know it. These are your mitochondria, aptly referred to as the “powerhouses” of the cell. They are primarily responsible for converting the food you eat into a usable form of energy stored in molecules called adenosine triphosphate (ATP).1
Your mitochondria produce about 90% of the energy your body requires to sustain life.2 This energy is necessary for every biological function, from thinking, breathing and moving to even unseen processes like immune defense and cellular repair.
According to the widely accepted endosymbiotic theory, over a billion years ago, mitochondria were once free-living bacteria that formed a mutually beneficial relationship with larger host cells. The bacteria contributed energy production through their metabolic processes, while the host cells provided a stable environment and access to nutrients. Over time, this relationship became permanent and evolved into the mitochondria we have in our cells today.3,4
There is still evidence of this evolutionary origin — mitochondria retain their own DNA, distinct from the DNA in the cell’s nucleus, allowing them to replicate independently. Structurally, mitochondria are also intricate. They are enclosed by a double membrane, with the outer membrane acting as a protective barrier, while the inner membrane folds into intricate structures called cristae.5
These folds dramatically increase the surface area available for energy production. Inside the inner membrane is the mitochondrial matrix, containing enzymes, mitochondrial DNA and ribosomes, which are all essential for maintaining cellular processes.6
Mitochondria are also remarkably adaptable. Their shape, number and location within cells change in response to energy demands. Cells that require more energy have higher concentrations of mitochondria.7 For instance, your brain accounts for only 2% of your body weight but consumes about 20% of your energy.8 Similarly, your heart, which beats over 100,000 times a day, relies heavily on mitochondrial activity to maintain its constant contractions.9
How Mitochondria Generate Energy

The mitochondria produce energy through a process called oxidative phosphorylation. This begins with glycolysis, which occurs in the cytoplasm, the jelly-like substance that surrounds the cell’s nucleus. During this stage, glucose is broken down into a simpler molecule called pyruvate, generating a small amount of ATP and NADH (nicotinamide-adenine dinucleotide), which carries energy.
The pyruvate is then transported into the mitochondrial matrix, where it undergoes pyruvate oxidation, forming acetyl-CoA. This process also produces more NADH and releases carbon dioxide. Acetyl-CoA enters the Krebs cycle (also known as the citric acid cycle), a series of chemical reactions that extract high-energy electrons from nutrients.
These high-energy electrons are carried by NADH and another molecule, FADH2, to the electron transport chain, a series of proteins located in the inner membrane of the mitochondria. As the electrons pass through the chain, they create a buildup of protons.
The protons flow back across the membrane through an enzyme called ATP synthase, which uses their movement to attach a phosphate group to ADP (adenosine diphosphate), turning it into ATP. Finally, oxygen plays a vital role as the last stop for the electrons in the chain. It combines with the electrons and protons to form water, a necessary byproduct that keeps the process running smoothly.10,11

Save This Article for Later – Get the PDF Now
What Causes Hiccups in Energy Production?
While oxidative phosphorylation is highly efficient, it’s not without flaws. An imbalance in the system leads to reductive stress, a condition where too many electrons accumulate in the electron transport chain. This often occurs when the chain slows down or when excessive NADH and FADH2 are produced, creating a cellular traffic jam.
One contributing factor to this imbalance is inadequate carbohydrate intake. Your body needs about 250 grams of carbohydrates daily to maintain a balanced energy production process. Without sufficient carbs, the body relies more heavily on fat metabolism, which generates higher levels of FADH2.
This overwhelms the electron transport chain, disrupting the smooth flow of electrons and reducing ATP production. When electrons get stuck, they interact with oxygen to form reactive oxygen species (ROS), which are unstable molecules that damage mitochondrial membranes, DNA and proteins.12
While small amounts of ROS are essential for cellular communication and defense, excessive ROS leads to oxidative stress, a condition that compromises mitochondrial function and energy production. This cascade results in inflammation, reduced energy levels and a host of chronic health problems.
Beyond Energy — The Multifaceted Role of Mitochondria
While ATP production is the mitochondria’s most well-known function, these organelles perform a range of essential roles that sustain cellular health and ensure proper physiological balance. These include:
• Calcium homeostasis — By acting as calcium reservoirs, mitochondria absorb and release calcium as needed, ensuring that intracellular calcium levels remain within optimal ranges. This regulation is essential for processes like muscle contraction, where calcium signals enable the precise control of muscle fibers.
In the nervous system, calcium fluctuations facilitated by mitochondria are vital for the release of neurotransmitters, which allow communication between neurons. Moreover, calcium regulation by mitochondria helps trigger apoptosis, ensuring that damaged or dysfunctional cells are safely removed without disrupting the surrounding tissue.13
• Apoptosis (programmed cell death) — In response to cellular damage, stress or infection, mitochondria release specific proteins, such as cytochrome c, which activate a cascade of molecular events leading to cell death.14
This tightly regulated mechanism prevents damaged cells from proliferating, which is essential for preventing chronic inflammation or cancer. Apoptosis also plays a vital role in development, such as shaping organs during embryogenesis, and in removing cells that are no longer needed, ensuring tissue homeostasis.15
• ROS signaling — Mitochondria produce reactive oxygen species as natural byproducts of cellular respiration. While excessive ROS leads to oxidative stress and damage cellular components, controlled amounts of ROS are essential for signaling and maintaining cellular health.
These molecules act as messengers, influencing pathways that regulate gene expression, immune responses and cellular adaptation to stress. For example, ROS signaling plays a role in triggering the body’s defenses against infections and facilitating tissue repair after injury.16
• Synthesis of metabolic intermediates — Mitochondria are also hubs for synthesizing metabolic intermediates required for various cellular processes. They contribute to the production of amino acids, the building blocks of proteins, which are essential for cell growth, repair and function.17
Mitochondria are also involved in lipid metabolism, including the synthesis of phospholipids like cardiolipin, which is vital for maintaining mitochondrial membrane integrity and functionality.18
Additionally, they contribute to the production of heme, a key component of hemoglobin, which allows red blood cells to transport oxygen throughout the body.19 These metabolic intermediates are indispensable for maintaining overall cellular and systemic health.
• Adaptability and cellular health monitoring — Mitochondria are dynamic organelles that continuously adapt to your cells’ energy and environmental needs.20 For instance, during periods of high energy demand, such as intense physical activity or recovery from injury, mitochondria rapidly increase ATP production to meet the cells’ needs.
Conversely, during times of stress or nutrient scarcity, they shift their metabolic focus to prioritize survival and repair processes. Mitochondria also serve as sensors of cellular health,21 detecting disruptions such as toxin exposure, oxidative damage or nutrient imbalances. In response, they initiate protective measures, activate repair mechanisms or, in extreme cases, trigger apoptosis to prevent further damage.
The Vital Role of ATP as an Energy Currency
Just like a car needs gas to run, your cells need ATP to fuel their processes. Without ATP, your cells will stop functioning, and so will you — that’s how essential it is. ATP is often called the “energy currency” of the cell. However, while this description captures its role in fueling biological processes, it only scratches the surface of ATP’s importance.
Structurally, ATP consists of a sugar molecule (ribose), a nitrogen base (adenine) and three phosphate groups. These phosphate groups are the key to ATP’s energy-storing capacity. The bonds connecting them are packed with potential energy, much like a coiled spring. When your body needs energy, it breaks one of these bonds, converting ATP into ADP and releasing a burst of energy that powers cellular processes.22
This process is akin to snapping the spring, unleashing its stored energy instantly. Your body is in a constant cycle of producing and using ATP. Each cell recycles its supply of ATP approximately every minute, generating an amount equal to your entire body weight daily.23
Most ATP production occurs through aerobic respiration in the mitochondria, which uses oxygen to efficiently generate energy. However, when oxygen is scarce, such as during intense exercise, ATP is produced anaerobically.24 This less efficient process generates lactic acid as a byproduct, causing the familiar burning sensation in your muscles.
ATP Plays Other Essential Functions in Your Body
While ATP’s primary role is to supply energy, its influence goes far beyond fueling cellular processes. ATP also acts as a signaling molecule, regulating numerous pathways to maintain cellular and systemic balance.
For instance, extracellular ATP binds to purinergic receptors on cell surfaces, setting off intracellular processes that influence cellular growth, differentiation, immune responses and tissue repair.25 This signaling helps your body adapt to changes, respond to damage and maintain overall homeostasis.
ATP also plays a role in moving ions like sodium, potassium and calcium in and out of cells.26 This keeps the right balance of these ions across cell membranes, which is essential for nerve signals, muscle movements and communication between cells.
Adaptation and survival under stress are also heavily reliant on ATP. When cells encounter environmental challenges or metabolic disruptions, ATP supports protective mechanisms such as the synthesis of specific heat shock proteins,27 antioxidants28 and DNA repair enzymes.29 These responses minimize damage and restore balance, particularly in conditions like oxidative or reductive stress.
In the brain, ATP plays a role in sustaining synaptic transmission and efficient neuronal signaling. The continuous production of ATP is essential to meet your body’s energy demands. When mitochondrial function declines, ATP production falters, leading to widespread energy deficits.
The Link Between Cellular Energy and Disease
Low mitochondrial energy production is the hidden driver behind most chronic diseases, affecting cellular function in ways that ripple throughout the entire body. This cascade of dysfunction it causes is akin to a car running on fumes — it may sputter along for a while but will eventually stop working altogether.
The relationship between mitochondrial dysfunction and disease becomes apparent when examining specific conditions. In diabetes, compromised mitochondrial function disrupts glucose metabolism, causing cells to become increasingly resistant to insulin.30
Pancreatic beta cells, which have exceptionally high energy demands to produce and secrete insulin, become overwhelmed and lose functionality.31 This creates a vicious cycle where energy deficits exacerbate metabolic dysfunction, making diabetes progressively harder to reverse.
Cancer represents another profound manifestation of disturbed cellular energetics. Cancer cells undergo a remarkable metabolic transformation known as the Warburg effect, where they shift away from efficient mitochondrial respiration toward increased glycolysis, even in the presence of oxygen.32
This seemingly counterintuitive change actually provides cancer cells with building blocks for rapid growth while helping them evade normal cellular death processes.33 Structural and functional abnormalities in the mitochondria of cancer cells further contribute to their aggressive behavior and resistance to treatment.
Neurodegenerative diseases also demonstrate the effects of energy deficit. The brain’s neurons, which require extraordinary amounts of ATP to maintain their complex networks and electrical signaling, fail to function properly as mitochondrial energy production declines.
This leads to the accumulation of toxic proteins, loss of calcium balance and, ultimately, neuronal death. This process manifests differently in various conditions — as memory loss and cognitive decline in Alzheimer’s disease, motor dysfunction in Parkinson’s disease and muscle weakness in amyotrophic lateral sclerosis (ALS).34
More About the Role of Energy Deficit in Illness and Aging
Mitochondrial dysfunction also drives autoimmune conditions. Immune cells require substantial energy for activation and proliferation, and mitochondrial dysfunction compromises their ability to function effectively. This leads to an overactive immune response, where immune cells attack the body’s own tissues, or to insufficient responses that fail to clear pathogens or debris.35,36
Cardiovascular disease, often viewed primarily through the lens of cholesterol and inflammation, has strong ties to mitochondrial dysfunction as well. Heart muscle cells house the highest density of mitochondria of any tissue, reflecting their constant energy demands. When mitochondrial function declines, the heart loses its ability to pump efficiently. This energy deficit manifest as heart failure, arrhythmias or increased susceptibility to ischemic damage.37
The aging process itself is intimately connected to declining mitochondrial function. As we age, mitochondria accumulate damage to their DNA, membranes and proteins. This deterioration creates a downward spiral where damaged mitochondria produce more harmful free radicals, leading to further damage.38
The decline in cellular energy production affects every aspect of aging, from reduced muscle strength and bone density to diminished cognitive function and immune response. This process accelerates the development of age-related conditions and compromises the body’s ability to maintain homeostasis.39
Chronic fatigue syndrome, once dismissed as purely psychological, has also emerged as a manifestation of mitochondrial dysfunction. Patients with this condition show measurable abnormalities in energy metabolism, with their cells struggling to produce adequate ATP even during rest.40
This lack of energy explains the extreme fatigue, worsening symptoms after activity and widespread issues seen in the condition. Without enough cellular energy, everything from muscle strength to brain function is affected, leading to symptoms that standard treatments typically cannot fix.
Mental health conditions, such as depression, anxiety and mood disorders, have strong ties to energy deficits as well.41 It makes sense when you think about how much energy your brain needs to make neurotransmitters, keep brain cells connected and manage the signaling networks that control mood and behavior.
Diagnostic Disconnect
Current medical approaches routinely fail to address the role of cellular energy production in health. Instead, they focus on treating symptoms, much like attempting to repair a car’s performance without checking if the gas tank is empty. This oversight leads to temporary fixes that don’t resolve the underlying problem, leaving patients stuck in a cycle of symptom management rather than true recovery.
In diabetes, for example, treatments typically aim to lower blood sugar levels without addressing the mitochondrial inefficiency that drives insulin resistance. While these interventions help control glucose levels, they don’t tackle the energy deficits that are central to the disease. Similarly, in neurodegenerative conditions, therapies target neurotransmitter imbalances but ignore the mitochondrial dysfunction that underlies cognitive decline.
The same disconnect exists in pain management. Chronic conditions like fibromyalgia are often treated with medications that dull symptoms but fail to restore the cellular energy systems required for long-term healing. This reliance on symptom suppression perpetuates dependence on pharmaceuticals while neglecting the opportunity for true healing.
Conventional medicine divides the body into isolated systems, treating each organ or function separately, which is a fool’s errand. This fragmented view of health reflects a broader issue — the failure to recognize the interconnectedness of the body’s systems and the foundational role of cellular energy production.
The Path Forward
Cellular energy is the vital yet overlooked link that modern medicine has ignored for far too long. Addressing it is not just a new approach — it is the only approach that delivers real, lasting results. Placing cellular energy at the core of every diagnosis and treatment plan redefines the medical paradigm, fundamentally transforming how we prevent and treat diseases.
This shift goes beyond managing symptoms or seeking short-term relief. It focuses on addressing the root cause — restoring your body’s innate ability to heal itself. Every cell in your body holds an incredible capacity to repair, regenerate and thrive, but it relies on one thing to function at its best — optimal energy. Without it, your health deteriorates and disease takes hold.
By prioritizing cellular energy, you unlock this remarkable ability to heal from virtually any disease. You no longer need to rely on temporary fixes from modern medicine that only mask the underlying problem. Instead, you build the foundation for health that is resilient, enduring and rooted in the natural design of your body.
This is a revolution in health and a return to what medicine was always meant to be — a system that supports the body’s ability to restore itself, not suppress it. The path forward is clear — it begins with cellular energy, the true foundation of lasting wellness.
- 1, 7 National Human Genome Research Institute, Mitochondria, January 22, 2025
- 2 Molecular and Cellular Endocrinology. Volume 551, 1 July 2022, 111661, Abstract
- 3 Antioxidants 2023, 12(4), 782, Mitochondria, the Key Aerobic Microbe for Eukaryotic Cell Evolution
- 4, 5 The Cell: A Molecular Approach. 2nd Edition., Mitochondria, The Genetic System of Mitochondria
- 6 Current Research in Physiology. Volume 4, 2021, Pages 163-176, Introduction
- 8 Current Opinion in Neurobiology. Volume 78, February 2023, 102668, Introduction
- 9 Biomolecules. 2024 Nov 29;14(12):1534
- 10 StatPearls [Internet]. Biochemistry, Anaerobic Glycolysis
- 11 The Cell: A Molecular Approach. 2nd Edition, The Mechanism of Oxidative Phosphorylation
- 12 Biomolecules 2024, 14(6), 670, Introduction
- 13 Antioxidants 2022, 11(5), 801, Introduction
- 14 The International Journal of Biochemistry & Cell Biology Volume 121, April 2020, 105704, Introduction
- 15 National Human Genome Research Institute, Apoptosis, January 22, 2025
- 16 Oxid Med Cell Longev. 2022 Oct 19;2022:1225578
- 17 Nat Rev Mol Cell Biol 22, 307–325 (2021)
- 18 Cells 2024, 13(7), 609, Introduction
- 19 Cells. 2020 Feb 29;9(3):579
- 20 Sig Transduct Target Ther 9, 124 (2024), Energy and Nutrient Sensing
- 21 Aging Cell. 2022 Nov;21(11):e13710
- 22 Britannica, Adenosine Triphosphate
- 23 Sci Adv. 2024 Nov 1;10(44):eadp7725, Introduction
- 24 StatPearls [Internet]. Biochemistry, Glycolysis, Fundamentals
- 25, 26, 29 StatPearls [Internet]. Physiology, Adenosine Triphosphate, Function
- 27 MedComm (2020). 2022 Aug 2;3(3):e161, HSPs Classification
- 28 Recent Trends in Pharmacology, 2(2), 79-82, Abstract
- 30 Front Physiol. 2019 May 3;10:532, Mitochondrial Dysfunction and Insulin Resistance
- 31 Life Sciences Volume 312, 1 January 2023, 121247
- 32 Advances in Experimental Medicine and Biology, May 2021; Vol 1311: Pages 3–15, The Warburg Effect
- 33 Front Immunol. 2021 Feb 2:11:621757
- 34 J Transl Med 21, 613 (2023), Mitochondrial Dysfunction in Neurodegenerative Disorders
- 35 Front. Immunol. 14:1304315
- 36 Biochimica et Biophysica Acta (BBA) – Molecular Basis of Disease Volume 1866, Issue 10, 1 October 2020, 165845
- 37 Antioxidants 2023, 12(4), 782, The Role of Mitochondrial Function in Multiple Diseases
- 38 Ageing Research Reviews Volume 88, July 2023, 101955
- 39 Front. Physiol. 15:1384966
- 40 J Transl Med 19, 81 (2021), Background
- 41 Schizophrenia Research Volume 273, November 2024, Pages 62-77
Cellular Health Revolution — Unveiling Hidden Threats and Empowering Solutions
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2025/02/02/cellular-health-revolution.aspx
Analysis by Dr. Joseph Mercola February 02, 2025
STORY AT-A-GLANCE
- In my appearance on The Jimmy Dore Show, we discussed how mitochondrial dysfunction, caused by modern toxins, is at the root of many diseases. ATP production has decreased by up to 75% compared to a century ago
- I shared insights from my latest book, “Your Guide to Cellular Health: Unlocking the Science of Longevity and Joy,” including that seed oils, like soybean and corn oil, are major culprits in damaging cellular health, while natural sugars can be beneficial when used wisely
- Endocrine-disrupting chemicals and EMFs from devices like cellphones and Wi-Fi routers pose significant risks to mitochondrial function, necessitating practical steps to reduce exposure
- Powerful foundations and industry interests have shaped medical education and public health policies, often prioritizing pharmaceutical interventions over natural approaches
- Restoring cellular health involves eliminating toxins, adopting a whole foods diet, optimizing sun exposure and addressing gut health imbalances
In my appearance on The Jimmy Dore Show, we explored a vital yet often overlooked aspect of human well-being — cellular health and the myriad of silent toxins eroding it. I shared insights from my latest book, “Your Guide to Cellular Health: Unlocking the Science of Longevity and Joy.”
This article reviews the most pivotal points from our extensive discussion, revealing the vital components that sustain health and the modern challenges that threaten them. As I shared with Dore, for 15 years I struggled with a mind-bending, unexplained rash that caused me to lose sleep at night because of unrelenting itching. All the physicians I consulted, some of the best out there, had no clue how to resolve it.
This personal battle led me to a groundbreaking realization: impaired mitochondrial function is at the heart of nearly every disease. Mitochondria, the powerhouses of your cells, produce adenosine triphosphate (ATP) — your body’s essential energy currency.
Everyone knows you can’t run a car without fuel. Similarly, your body is a vehicle that transports you around, and if you don’t have enough energy, it’s a problem. Historically, humans produced twice the amount of ATP compared to today, but the influx of chemical toxins has drastically reduced cellular energy production, leading to a significant decline in overall health.
The ATP Crisis — A Modern Epidemic
Humans are producing up to 75% less ATP today than a century ago. This decline is not just a number — it’s a reflection of our deteriorating health. The question remains: Why has ATP production plummeted?
The answer lies in the toxins that have permeated our environment over the past 150 years. The Industrial Revolution and subsequent advancements introduced chemical poisons into our lives, fundamentally disrupting our cellular machinery. Among these toxins, seed oils like soybean, corn and sunflower oil, stand out as primary culprits in harming your cellular energy.
Seed Oils — The Silent Destroyers of Health
Seed oils rich in polyunsaturated fatty acids (PUFAs) are one of the main drivers destroying your health, as excess consumption leads to obesity, diabetes, heart disease, cancer and dementia. These oils, often misleadingly labeled as “healthy” vegetable oils, wreak havoc on mitochondrial function. Consuming excessive amounts overloads your cells with harmful fats, crippling their ability to produce ATP.
Safe alternatives include coconut oil, ghee and beef tallow. Eating out poses a significant challenge for those striving to avoid seed oils like canola and soybean oil. One practical tip when you dine at a restaurant is to inform the server that you have a severe allergy to seed oils. Show them evidence of the dangers, and ensure the kitchen adheres to your requirements.
Most restaurants are unaware of the extent of seed oil contamination. By educating them and insisting on pure fats, you protect your health while raising awareness. The prevalence of adulterated oils, even in the case of products like extra virgin olive oil, makes vigilance essential. Additionally, cooking your own meals at home or choosing restaurants that use healthier frying fats, such as beef tallow, will significantly reduce your exposure to harmful PUFAs.

Save This Article for Later – Get the PDF Now
The Truth About Sugar — A Cellular Fuel
Contrary to popular belief, not all sugars are detrimental. Sugar, when used wisely, restores your energy. The key lies in understanding the type of sugar and its role in your metabolism. Real sugar — specifically glucose, also known as dextrose — is the ultimate fuel for your mitochondria. Unlike high-fructose corn syrup, which is harmful, glucose is essential for efficient energy production.
However, moderation is crucial. If you consume too much sugar, it disrupts insulin and hormonal balance. For individuals suffering from severe mitochondrial poisoning, however, glucose is a lifesaver, providing the necessary energy to sustain vital bodily functions. This nuanced understanding of sugar’s role challenges the conventional narrative that all carbohydrates are harmful.
Your Gut Microbiome — Balancing Good and Bad Bacteria
Your gut health plays a pivotal role in cellular energy and overall well-being. I explained the importance of colonocytes — cells lining your colon that rely on short-chain fatty acids like butyrate, propionate and acetate, produced by beneficial bacteria. When mitochondrial function is impaired, these colonocytes begin to die, allowing oxygen to seep back into your gut.
This shift creates an environment where pathogenic, oxygen-tolerant bacteria thrive, producing endotoxins that further damage mitochondria. This creates a vicious cycle I call the “black hole of death.”
To break free from this cycle, it’s essential to restore the balance of gut bacteria. Unfortunately, many people are unaware of the state of their microbiome, as beneficial bacteria are often overshadowed by their pathogenic counterparts. Advanced testing, though expensive, provides insights into the state of your gut health, but practical dietary adjustments are equally important.
Until an intervention targets the factors harming your colonocytes and restores optimal oxygen levels, the population of beneficial, oxygen-intolerant microbes cannot be reestablished in your gut. This mitochondrial-gut microbiome communication is necessary for health. However, when oxygen-tolerant pathogenic bacteria dominate, they outcompete the beneficial, oxygen-intolerant bacteria, preventing the production of essential metabolites needed for vitality.
Removing excess oxygen from your colon is key because, without it, even the best lifestyle practices — such as exercise, adequate sleep proper nutrition and the use of supplements — will not result in significant improvement. Simply introducing probiotics is insufficient for replenishing oxygen-intolerant microbes, as most commercially available probiotics are often non-viable.
While these probiotics offer postbiotic benefits, they do not function as true “seeds” to rebuild the appropriate microbial community. Just as a seed cannot grow in a desert, the environment within your colon must be conducive for these beneficial microbes to flourish.
Therefore, avoiding mitochondrial poisons, including seed oils and endocrine-disrupting chemicals, is essential to create the right conditions for restoring a healthy, oxygen-intolerant microbial population in your gut.
The Hidden Dangers of Plastics and Endocrine Disruptors
Beyond dietary choices, environmental toxins like plastics pose a significant threat to cellular health. Plastics produce endocrine-disrupting chemicals (EDCs) that stimulate estrogen receptors. These chemicals are pervasive, found in everyday items like water bottles and food wraps, and are linked to various health issues, including breast cancer.
EDCs disrupt hormonal balance, leading to widespread health problems. These chemicals primarily operate by activating estrogen receptors within your cells. This activation leads to an increased influx of calcium ions into your cells. Excessive intracellular calcium dramatically elevates the levels of superoxide and nitric oxide.
These reactive molecules swiftly combine to form peroxynitrite, an extremely potent oxidant stressor. The formation of peroxynitrite induces severe oxidative stress, resulting in significant cellular damage. In addition, when combined with natural estrogen, exposure to EDCs leads to estrogen overload and initiates a series of harmful events.
How EDCs Trigger Your Self-Attack Autoimmune Responses
Endocrine-disrupting chemicals (EDCs) pose a significant threat to your health, initiating a cascade of negative effects that begin at the cellular level and ripple outward to impact your entire body. This process unfolds in several interconnected stages, each building upon the last to create a perfect storm of health challenges in your system.
It all starts with your mitochondria — the powerhouses of your cells. EDCs interfere with these crucial organelles, diminishing their ability to produce the energy your cells need to function optimally. This energy deficit isn’t just a matter of you feeling tired; it has far-reaching consequences, particularly for your gut health.
Your digestive system relies on a delicate balance of beneficial bacteria, many of which thrive in an oxygen-free environment. The energy shortage caused by mitochondrial dysfunction disrupts this carefully maintained anaerobic setting in your gut. As a result, these beneficial microorganisms struggle to survive and perform their vital functions within you.
One of the key roles of these gut bacteria is the production of short-chain fatty acids. These compounds are essential for maintaining the health and integrity of your intestinal lining. They act as a primary food source for the cells that make up this barrier and help regulate the immune responses in your gut. However, when your gut bacteria are compromised due to the altered environment, their ability to produce these crucial fatty acids is severely impaired.
The absence of adequate short-chain fatty acids leads to a weakening of your intestinal barrier. This condition is often referred to as “leaky gut” or increased intestinal permeability. In this state, the tight junctions between the cells lining your intestines become loose, allowing substances that should remain within your gut to pass into your bloodstream.
This is where the situation can take a particularly concerning turn for you. Among the substances that can now penetrate your weakened gut barrier are proteins that bear a striking resemblance to structures within your own body — such as those found in your joints or neurological tissues.
When these foreign yet familiar proteins enter your bloodstream, your immune system is faced with a case of mistaken identity. It perceives these proteins as threats and mounts an attack against them.
The problem is, due to the similarity between these intruding proteins and your own body tissues, your immune response doesn’t stop at neutralizing the perceived invaders. Instead, it can turn against your own cells and tissues that share similar structures. This misdirected immune attack is the hallmark of autoimmune diseases, where your body essentially wages war against itself.
Thus, from the initial disruption of cellular energy production by EDCs, you arrive at a situation where your body’s own defense mechanisms have been tricked into causing harm to you. This complex chain of events underscores the far-reaching and interconnected nature of your body’s systems and highlights how these seemingly small disruptions can cascade into significant health challenges for you.
The challenge lies in finding safe alternatives, as conventional plastics are laden with harmful chemicals. I’m in the process of creating bio-compatible alternatives to plastics in order to help eliminate EDC exposure and promote environmental sustainability.
Electromagnetic Fields (EMFs) — A Mitochondrial Poison
Another insidious threat to your cellular health today is exposure to EMFs. Unlike other toxins, EMFs permeate our environment, making them a pervasive danger that is often overlooked.
Electromagnetic frequencies describe all types of radiation, including beneficial ones like sunlight. However, the high-frequency EMFs emitted by modern devices such as cellphones, Wi-Fi routers and microwaves operate in the gigahertz range, posing significant risks to your mitochondria.
While ionizing radiation like X-rays directly damages cells by creating free radicals, EMFs cause harm through a different mechanism called non-thermal effects. These non-thermal effects disrupt cellular function without raising tissue temperatures, making the damage less visible but equally, if not more, dangerous.
EMFs interfere with mitochondria by increasing calcium ion influx into cells. Elevated calcium levels catalyze the production of harmful free radicals, leading to oxidative stress and mitochondrial dysfunction. This process mirrors the damage caused by other mitochondrial poisons, like seed oils and EDCs, creating a vicious cycle of cellular decline.
The Telecommunication Industry’s Deceptive Practices
The telecommunications industry, much like the tobacco industry before it, has employed deceptive strategies to downplay the dangers of EMFs. They used the same playbook as the tobacco industry to greenwash their products and obfuscate the real risks. The 1996 Telecommunications Act, for instance, effectively immunized these companies from liability, allowing them to continue disseminating harmful EMFs without accountability.
They promote the idea that non-ionizing radiation is safe because it doesn’t cause immediate thermal damage. This misleading narrative ignores the long-term, chronic effects of EMF exposure, which accumulate over time and contribute to a host of health problems, including cancer, neurological disorders and reduced cellular energy.
Practical Steps to Mitigate EMF Exposure
Understanding the dangers of EMFs is only the first step; taking actionable measures to reduce exposure is crucial for safeguarding your health. Here are several strategies I recommend:
1. Limit cellphone use — Avoid keeping your cellphone close to your body, especially when sleeping. Cellphones emit high levels of EMFs and prolonged exposure significantly disrupts mitochondrial function. I personally use an EMF shield tent to create a low-radiation environment during sleep, ensuring that my mitochondria remain untainted by these frequencies.
2. Reduce Wi-Fi dependency — Turn off Wi-Fi routers when not in use, especially at night. Wi-Fi is a constant source of EMFs in many households, and minimizing its operation drastically reduces overall exposure. For essential connectivity, use wired Ethernet connections instead of wireless alternatives.
3. Create EMF-free zones — Designate certain areas of your home, such as your bedroom, as EMF-free zones. By establishing a sanctuary free from electromagnetic radiation, you provide your mitochondria with the environment they need to function optimally. Simple steps like using wired devices and keeping electronic gadgets out of these areas makes a significant difference.
4. Use EMF shielding products — Investing in EMF shielding products, such as EMF-blocking phone cases or shielding tents, provides additional protection. These products help deflect or absorb harmful frequencies, safeguarding your cellular health. While not a complete solution, they offer a practical layer of defense against unavoidable EMF exposure.
Reclaiming Health — Strategies for Protection and Restoration
To break free from this cycle of mitochondrial poisoning, it is imperative to adopt comprehensive strategies that eliminate exposure to harmful toxins and support mitochondrial function. Here are several actionable steps:
1. Eliminate seed oils and processed foods — As mentioned, seed oils like soybean, corn and sunflower oil are laden with PUFAs that oxidize easily, producing toxic metabolites that damage mitochondria. By removing these oils from your diet, you reduce the primary source of mitochondrial poisoning.
2. Adopt safe fats and whole foods — Incorporate saturated fats such as coconut oil, butter, ghee and beef tallow into your diet. These fats are stable and support mitochondrial function without the harmful effects of PUFAs. Additionally, focus on whole, unprocessed foods that provide essential nutrients without the added toxins found in processed products.
3. Minimize EMF exposure — Limit your exposure to EMFs by reducing the use of wireless devices and turning off Wi-Fi when not in use. Embracing EMF shielding solutions, such as EMF-blocking phone cases and creating EMF-free zones in your home, significantly reduces cellular stress and supports mitochondrial health.
4. Optimize sun exposure — Embrace sun exposure around solar noon once you have eliminated seed oils from your diet. Proper sun exposure enhances mitochondrial energy production and supports overall health. Use minimal, protective clothing to maximize benefits while preventing skin damage.
5. Restore gut health — Addressing gut microbiome imbalances is crucial for maintaining cellular energy. Focus on consuming beneficial bacteria and limiting fiber intake if pathogenic bacteria dominate your gut. Probiotic supplements and dietary adjustments help restore a healthy balance of gut flora, supporting mitochondrial function.
The Power of Education and Advocacy
Raising awareness about the true impact of these mitochondrial poisons is essential for empowering individuals to take control of their health. Education and advocacy are crucial in countering the misinformation spread by powerful industries. By informing the public about the real dangers of seed oils, EMFs and mask mandates, we foster a movement toward healthier living and systemic change.
On an individual level, you must take proactive steps to protect your health, even in the face of pervasive EMF exposure and misleading public health directives. Simple lifestyle changes, combined with a commitment to natural health principles, significantly enhance mitochondrial function and overall well-being.
A Vision for a Healthier Future
Looking ahead, my mission is to continue developing solutions that protect and restore cellular health. These efforts aim to harmonize technological advancements with natural health practices, ensuring that progress does not come at the expense of our well-being.
The goal is to empower individuals with the knowledge and tools needed to maintain robust mitochondrial function and achieve lasting health. By addressing the root causes of mitochondrial poisoning and advocating for informed, holistic health practices, we’ll can pave the way for a future of longevity and joy.
Relationship Found Between Creatine in the Brain and Recovery From Traumatic Stress
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2024/07/17/creatine-brain-benefits.aspx
Analysis by Dr. Joseph Mercola July 17, 2024

STORY AT-A-GLANCE
- While creatine is well-known for its benefits in physical performance and muscle health, its potential advantages for brain health are becoming increasingly recognized
- Creatine, which is naturally found in muscle cells and the brain, may assist in recovery from the stress of traumatic experiences
- Creatine is used by your body to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP) — the main energy currency of cells — and may be a neurobiological marker of stress reactivity and recovery
- Veterans with higher levels of creatine in the anterior cingulate cortex — a brain region involved in processing negative emotional states — had greater reductions in stress since their last traumatic experience
- Creatine is found only in animal-based food — not plants — as well as in supplement form; grass fed meats are among the best sources of creatine, but avoid pork and chicken, as they typically have high levels of linoleic acid
Creatine is a substance naturally found in muscle cells and the brain. It helps your muscles produce energy during high-intensity exercise or heavy lifting. Most of the body’s creatine is stored in muscles, where it’s used for quick bursts of energy.
As such, creatine is commonly used by athletes to improve performance, as it’s immediately used by your body to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP) — the main energy currency of cells — and supply energy muscles need for contraction. However, creatine also helps provide energy to your brain and research suggests it may play an important role in recovery from traumatic stress.
Unraveling the Neurobiological Reasons Why Some Develop PTSD — and Others Don’t
While creatine is well-known for its benefits in physical performance and muscle health, its potential advantages for brain health are becoming increasingly recognized. In a study involving U.S. veterans, researchers with the University of Utah School of Medicine revealed the compound may assist in recovery from the stress of traumatic experiences.
“There is emerging preclinical evidence that creatine (Cr), a molecule critical to brain bioenergetics, may be a neurobiological marker of stress reactivity and recovery,” the scientists wrote in the Journal of Affective Disorders.1 They noted that little is known about why some individuals recover from traumatic events while others develop post-traumatic stress disorder (PTSD) and other psychological conditions.
The symptoms of PTSD can be categorized into four main types: intrusive memories, avoidance, negative changes in thinking and mood, and changes in physical and emotional reactions. These symptoms can vary over time and differ from person to person.
According to the study, about 70% of adults worldwide have experienced at least one traumatic life event. Yet, the lifetime prevalence of PTSD is 6.1%, “implying that most individuals exhibit robust recovery from traumatic life events.” The researchers explain:2
“This line of research suggests that individual differences in the stress recovery process may be involved in the pathogenesis of PTSD. Thus, understanding factors associated with recovery from traumatic life events may provide novel insights into the assessment, prevention and treatment of PTSD and other trauma-related conditions.”
While early childhood experiences, individual personality and the number of traumatic experiences a person has likely play a role, neurobiological factors may also be involved. Toward this end, the anterior cingulate cortex (ACC) is a brain region involved in processing negative emotional states.3 Located in the frontal lobe, the ACC plays a critical role in various cognitive and emotional processes.
The ACC is particularly active during experiences of negative emotions, such as pain, sadness and fear. It’s also involved in the regulation of emotional responses, decision-making and the anticipation of adverse outcomes. The researchers wondered if neurochemical factors were also involved, leading them to creatine:4
“Existing research suggests that Cr concentrations in the ACC are indicative of stress-related mental health conditions, which are often precipitated by traumatic life events. Moreover, preclinical models in animals indicate exposure to traumatic stress reduces ACC Cr levels. Nevertheless, it remains unclear to what extent traumatic life events in humans are associated with Cr concentrations in the brain.”
Creatine May Help Recovery From Traumatic Stress
To help reveal the relationship between creatine concentrations in the ACC and stress related to traumatic life events, the researchers conducted brain scans on 25 U.S. veterans and collected data on their mental health status and history of traumatic events.
Veterans with higher levels of creatine in the ACC had greater reductions in stress in the time since their last traumatic experience.5 “ACC concentrations of Cr may be an important neurochemical factor related to stress recovery. Future work should investigate Cr as a possible protective factor against the effects of traumatic stress,” the study concluded.6
Again, creatine enables ATP regeneration, which plays a crucial role in cellular function. And, as PsyPost noted, “The researchers hypothesized that creatine levels in the brain could influence an individual’s ability to recover from trauma by affecting the energy availability in critical brain regions.”7
This is an example of why you need ample cellular energy for optimal brain function. Your brain, being the most energy-dependent organ, makes up only about 2% of your bodyweight yet consumes 20% of the energy used by your entire body.8 Therefore, a surplus of cellular energy creation is necessary to have the ability to allow your brain to work optimally.
Without enough cellular energy, not only is your ability to think and make good decisions compromised, but mental health is subsequently compromised as well.

Save This Article for Later – Get the PDF Now
How to Increase Cellular Energy
Avoiding dietary pitfalls like excess linoleic acid (LA), in the form of vegetable and seed oils, is instrumental in optimizing mitochondrial function and increasing cellular energy. Factors like estrogen and endotoxins can also deplete your cellular energy. Meanwhile, improving your mitochondrial energy production can also help bring you joy, which is essential for mental health.
Creatine, however, is also important. By supporting ATP regeneration, creatine helps improve the efficiency of energy utilization in cells. Creatine may also support mitochondrial function by enhancing the availability of ATP, improving overall cellular energy metabolism.
The following are key concepts that need to be integrated to improve all cellular energy, and certainly energy produced for your brain. They all revolve around improving mitochondrial function:
- Lower LA as much as possible — This is the single most important mitochondrial poison. Since there’s no downside to limiting your LA, you’ll want to keep it as low as possible, which you do by avoiding high-LA foods, including vegetable and seed oils found in most ultraprocessed foods.
- Lower estrogen excess — Estrogen, even bioidentical, is nearly as dangerous as LA in destroying mitochondrial function. Aside from avoiding all estrogen supplements and plastics, as they are potent sources of xenoestrogens, you can take trans mucosal progesterone, not oral or transdermal, as it is a potent estrogen blocker.
- Make sure your thyroid is working well — Thyroid function is absolutely essential to make sure you have a high metabolic rate and produce plenty of ATP. If you are going to do a thyroid test, it is important your TSH be well-suppressed and below 0.5. You can also confirm by taking your temperature first thing in the morning and two hours after meals. Low temperatures indicate low thyroid activity.
- Optimize your microbiome — This is also key, as not only are 95% of people metabolically inflexible, but because of mitochondrial poisons their microbiome is out of balance with a preponderance of pathogenic endotoxin-producing bacteria, another potent mitochondrial poison.
Creatine’s Many Brain Benefits
Stuart Phillips, Ph.D., is a professor of kinesiology at McMaster University in Canada. He’s an expert in growing and maintaining muscle mass as you age. In an interview with Rhonda Patrick, Ph.D.,9 he commented on using creatine regularly, “You know, the stuff now with creatine that they’re uncovering that makes me think, ‘Maybe this should be part of my regular routine.’ Actually [it] has less to do with the muscle and more to do with the brain and cognitive performance.”
In fact, creatine monohydrate is one supplement that Phillips said makes his short list for its benefits for muscle growth and brain health. “Its effects are pretty mild on muscle, but they’re there. They’re potent. They last. Now the brain and the cognitive side of things … the evidence is growing in that area too.”10
Research has demonstrated that creatine plays a critical role in the function of the brain and other tissues that have high energy demand.11 Children who have genetic errors of creatine synthesis present with severe neurological symptoms and patients with other neurodegenerative diseases benefit from creatine supplements. Other research suggests reduced creatine is associated with depression and anxiety.12
Creatine also has demonstrated beneficial effects in mice with Parkinson’s-like disease.13 It prevented 90% of the typical drop in dopamine levels that are associated with several of the serious symptoms, including loss of muscle function and speech impairment.
In an experimental model mimicking the effects of a mild traumatic brain injury, one research team even found supplementing with creatine helped cognitive processing during oxygen deprivation.14 They concluded, “This is the first demonstration of creatine’s utility as a neuroprotective supplement when cellular energy provision is compromised.”
Other research, published in Nutrition Reviews, found creatine supplementation enhanced memory performance in healthy adults, particularly those aged 66 to 76 years.15 The study concluded:16
“These beneficial effects from creatine supplementation on memory performance may be related to creatine’s ability to influence brain bioenergetics. For example, creatine elevates phosphocreatine and ATP levels and increases oxidative phosphorylation in synaptosomes and isolated brain mitochondria. In hippocampal neuron cultures, creatine stimulates mitochondrial activity.”
The Best Sources of Creatine
For individuals looking to increase their creatine intake through diet, including a variety of creatine-rich foods can be beneficial. Creatine is found only in animal-based food — not plants. Grass fed meats are among the best sources of creatine. Avoid pork and chicken, as they typically have high levels of LA.
While grass fed meats can contribute to creatine intake, they may not provide the same high doses that can be achieved through creatine supplementation. Of the different formulations of creatine on the market, creatine monohydrate is the one that has been studied most frequently and therefore has the strongest evidence of health benefits.
It’s important to choose creatine from a reputable manufacturer. Clinical trials that have lasted up to five years have reported no adverse effects in healthy individuals.17 However, it is important to stay within the recommended dose.
Some people are sensitive to using creatine and feel bloated if they don’t drink enough water with the supplement. However, most of the time it goes away in just a few hours. Factors that affect bloating include how much water you drink, the intensity of your workouts and your diet.
If you’re a vegan or a vegetarian, you might consider using creatine to help protect brain health. Research suggests creatine supplements may boost cognitive function in vegetarians.18
- 1 Journal of Affective Disorders June 15, 2024, Volume 355, Pages 115-121
- 2, 3, 4, 12 Journal of Affective Disorders June 15, 2024, Volume 355, Pages 115-121, Introduction
- 5, 7 PsyPost May 13, 2024
- 6 Journal of Affective Disorders June 15, 2024, Volume 355, Pages 115-121, Abstract
- 8 Cell Reports May 29, 2018
- 9 YouTube, FoundMyFitness June 29, 2022, 1:47:33
- 10 YouTube, FoundMyFitness June 29, 2022, 1:44
- 11 Annual Review of Nutrition, 2007; 27
- 13 Experimental Neurology, 1999;157(1)
- 14 The Journal of Neuroscience, 2015;35(4)
- 15 Nutrition Reviews, Volume 81, Issue 4, April 2023, Pages 416–427
- 16 Nutrition Reviews, Volume 81, Issue 4, April 2023, Pages 416–427, Discussion
- 17 Journal of the International Society of Sports Nutrition, 2007;4(6) Medical Safety of Creatine Supplementation para 2, 3
- 18 Science Daily April 8, 2019
Kidney Stones in Children Are Becoming More Prevalent — Here’s Why and How to Fight Them
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2024/05/16/kidney-stones-in-children.aspx
Analysis by Dr. Joseph Mercola May 16, 2024

STORY AT-A-GLANCE
- Cases of kidney stones among children are increasing, and although less common than in adults, it could become a lifelong battle
- Oxalates, which are found in many plant foods, are a contributing factor to the rising cases of kidney stones. When these compounds bind with calcium, they form calcium oxalate crystals, which are microscopic and razor-sharp, and can cause significant tissue damage
- By optimizing your metabolic flexibility, you can maintain a low-oxygen environment in your gut. This allows healthy obligate anaerobes, which can help metabolize and eliminate oxalates, to thrive
- Removing high-oxalate foods from your diet is the first step to minimize their harmful effects and help heal your gut. Some strategies that can aid in eliminating oxalates are also discussed here
Kidney stones are hard masses that form from the chemicals in the urine when there’s too much waste and too little liquid. They can be as small as a grain of sand, or as big as a pebble — in some cases, they can grow as large as a golf ball. As your body works to eliminate the stone, it can lead to irritation or blockage, causing intense pain and other symptoms.1
In adults, kidney stones are a common health complaint, with 8 out of 1,000 adults being diagnosed yearly.2 Alarmingly, cases among children are increasing as well.
Is Your Child at Risk of Developing Kidney Stones?
According to an article in ABC7,3 kidney stones have become more prevalent in children over the last 20 years. Although less common than in adults, it could still be a lifelong battle. The article tells the story of Alex Zellers, a 4-year-old with a rare genetic disease called cystinuria, which caused him to develop enlarged kidney stones that had to be surgically removed.4
“One stone in his kidney was the size of a golf ball. The other, in his bladder, was the size of a lacrosse ball. ‘It’s just like a giant dense egg. It’s just a big mass,’ described Kate, Alex’s mother.”
In the article, Dr. Greg Tasian, a pediatric urologist with Children’s Hospital of Philadelphia, explains how kidney stones form, saying “Your body doesn’t absorb certain amino acids and that cystine accumulates and crystallizes in the urine forming stones early in life.”
And although Alex’s condition is rare, Tasian claims that he is seeing an increase in young patients with kidney stones and says that several lifestyle factors are to blame, such as eating more ultraprocessed foods, excessive of use antibiotics and being chronically dehydrated, especially during hot weather.5
However, there could be another more significant contributing factor, and it’s found in the foods you eat — even those that are considered healthy.
Oxalates Are Linked to Kidney Stones, but What Are They?
Oxalates are natural compounds found in many plant foods, including beans, grains, seeds and nuts, fruits, berries and herbs.6 They’re also called dicarboxylic acid, meaning they are composed of two carbon dioxide (CO2) molecules.
However, having two carboxyl groups (COOH), causes them to lose protons under physiological conditions. This leaves them with a negative charge, which then allows them to bind to positively charged ions like calcium.
Chemically, oxalate is a salt; and as with other salts, it forms crystals that your body innately has a limited capacity to process. When oxalates bind with calcium, they form calcium oxalate crystals, which are microscopic and razor-sharp and can cause significant tissue damage. And because they are not soluble, they can accumulate.
This is what causes kidney stones to form. Calcium stones are the primary type, making up 80% of kidney stones.7 But contributing to the formation of kidney stones is just one of the ways oxalates wreak havoc on your health. These compounds can affect numerous body functions and cause a wide range of symptoms.

Save This Article for Later – Get the PDF Now
A High-Oxalate Diet Can Lead to Joint Pain, Digestive Problems and Skin Irritation
Excessive oxalates can affect your absorption of essential nutrients and lead to mineral deficiencies. When they accumulate in your joints, they can cause crystals, similar to those in the kidneys, to form. This can trigger inflammation and joint pain, resembling symptoms of gout or arthritis.
In your urinary tract, oxalates can cause irritation, discomfort and an increased risk of urinary tract infections (UTIs). The razor-sharp crystals can also make urination painful, and contribute to irritable bladder syndrome, which is characterized by frequent, urgent and/or painful urination.
Meanwhile, bloating, gas, diarrhea and abdominal pain can arise when oxalates affect your intestinal tract, especially in people with sensitive digestive systems or who have irritable bowel syndrome (IBS).
There’s also research linking a high-oxalate diet with fibromyalgia symptoms, and while still not fully understood, the theory is that oxalate crystals may be causing inflammation in the muscles and connective tissues, causing widespread pain and fatigue.
Once your body tries to eliminate oxalates, they can be excreted through your skin, particularly if your kidneys can no longer process the excessive amounts of oxalates in your system. This can form crystalline deposits on your skin, causing irritation, rash and intense itching.
I struggled with this health problem 15 years ago, when I developed a rash that caused such intense itching it made me lose sleep. When scratched, the rash would turn into hard nodules that would last for months or years.
I tried numerous natural interventions, including icing the affected area and applying aloe gel, but could not find any long-lasting solution — it was only when I addressed the oxalates in my diet that I was able to find relief.
Oxalates Can Interfere With Your Cellular Functions
Another way that oxalates harm your health is by disrupting enzyme functions that are essential to cellular energy production. Oxalate ions can bind to the enzymes in the mitochondrial electron transport chain, which are essential for adenosine triphosphate (ATP) production.
Your mitochondria produce ATP, which is why they are called the “powerhouses” of your cells. ATP is the currency of your cellular energy and is the lifeblood of cellular processes. It powers everything, including processes like muscle contraction, nerve impulse propagation, synthesis of essential biomolecules and the maintenance of cellular homeostasis.
When oxalates disrupt ATP production, it can lead to decreased energy production and increased oxidative stress within cells. This then leads to a broader range of metabolic and physiological dysfunctions.
Avoid These High-Oxalate Foods
Everyone needs to be concerned about oxalates, not just those dealing with kidney stones or other chronic health issues, metabolic inflexibility or mineral imbalances. The first step is to identify high-oxalate foods and remove them from your diet, until your gut is healed.
I recently interviewed Sally Norton, who is an esteemed authority on oxalates. Her expertise is indeed invaluable for anyone seeking to understand this topic. In our discussion, she specified the foods that are particularly loaded with oxalates. You may be surprised, as some of these are on many people’s “healthy foods” list:
• Spinach — Their oxalate levels can be as high as 600 to 800 mg per 100 grams.
• Almonds — Almonds generally contain about 122 mg of oxalates per 100 grams. However, all nuts in general are particularly problematic, since they contain linoleic acid (LA). Even macadamia nuts can add to your toxic load, as they contain oleic acid, which could just be as bad as LA.
“These seeds from the trees are designed with all these multiple anti-nutrients to kick you in the gut. All the anti-nutrients are gut toxic. They’re all causing some degree of gut damage. Nuts are just designed to be indigestible. They’re designed to dismantle your ability to digest food. If you want a healthy gut, you don’t want nuts kicking your gut over and over again,” says Norton.
• Peanut butter — Peanut butter can have around 140 mg per 100 grams.
• Sweet potatoes — They contain about 30 mg of oxalates per 100 grams. (Although this is considered high compared to other vegetables, it’s actually much lower than spinach or nuts)
• Figs — They have approximately 40 mg per 100 grams.
In addition to spinach, high-oxalate leafy greens that are considered “superfoods” are Swiss chard and beet greens, so you may want to avoid them if you’re sensitive to oxalates or are struggling with kidney stones.
You may also want to avoid these collagen-rich protein sources until your gut is healed, as collagen breakdown can lead to oxalate production and aggravate your condition:
- Bone broth
- Gelatin
- Animal skins, tendon and ligaments
- Meat cuts with connective tissues such as oxtail, neck and shank
- Organ meats like heart and liver
Healing Your Gut Can Help Address Oxalate Toxicity
I mentioned above that healing your gut health is crucial to help curb the effects of oxalates, but before you do that, you need to address your metabolic inflexibility. This refers to your body’s diminished ability to switch between burning fuel sources, mainly carbohydrates and fats.
When you’re metabolically inflexible, it can affect your body’s ability to produce energy. This can have a profound impact on your gut health, particularly your large intestine, as it hinders your body’s ability to maintain a low-oxygen environment in this organ.
You need a low-oxygen environment in your large intestine because not only does it help keep pathogenic bacteria in check, but it also allows healthy obligate anaerobes to thrive. These are a primitive type of bacteria that cannot survive when exposed to oxygen.
So what do obligate anaerobes have to do with oxalate toxicity? It turns out that there are obligate anaerobes that can digest oxalate crystals, called Oxalobacter formongines.8 These beneficial bacteria thrive in a low-oxygen environment and have a unique ability to efficiently metabolize oxalates.
Using specific enzymes, Oxalobacter bacteria break down oxalate crystals into formate and carbon dioxide. The carbon dioxide then helps retain the low-oxygen environment in your intestine, allowing these primitive organisms to thrive and support your health. Through simple passive diffusion, the crystals are released and wind up in your intestine where the Oxalobacter continues to digest them until the oxalate toxicity issues disappear.
To put it simply, you need to optimize your metabolic flexibility so you can maintain a low-oxygen environment in your gut and allow Oxalobacter bacteria to radically reduce the level of oxalates in your tissues.
I believe this is the ultimate cure for most kidney stones. It’s far more efficient and effective than the conventional approach for this common health condition, as it goes straight to the root cause of the problem.
Step 1 in healing your gut would be to eliminate linoleic acid (LA) from your diet, as LA precipitates the formation of peroxynitrites that ravage mitochondrial function and impede energy production, forcing your body to rely on glycolysis in the cytoplasm of your cells rather than the electron transport chain (ETC) of your mitochondria.
This, in turn, results in the impairment of your gut by allowing oxygen leakage into your gut that kills beneficial bacteria and allows pathogenic bacteria to thrive.
There’s No Quick Way to Detox Oxalates
If you or your children struggle with kidney stones or have other signs of oxalate toxicity, I encourage you to watch my interview with Norton, as we discuss many strategies and food choices that can help minimize the harmful effects of oxalates or aid in their elimination.
Aside from limiting your intake of high-oxalate foods mentioned above, here are some key recommendations to remember:
| Increase your calcium intake — When you consume foods high in calcium or take calcium supplements, they can bind to oxalates in the intestines and prevent them from being absorbed. They will also help facilitate oxalate excretion through your stool. Foods rich in calcium include dairy products and leafy greens. |
| Stay hydrated — Drinking sufficient water will help flush out oxalates through your urine and keep kidney stones from forming. |
| Optimize your gut health — Promote a healthy gut microbiome by consuming probiotic-rich foods like yogurt, kefir and fermented vegetables. This will help support the growth of Oxalobacter and other beneficial bacteria. |
| Citrate consumption — Citrate, found in citrus fruits like lemons and oranges, can help by binding with calcium and oxalate, thereby reducing the formation of kidney stones. Avoid over-supplementation with ascorbic acid, however, as high doses can convert into oxalate. Ascorbic acid is the most common form of vitamin C used in dietary supplements. |
| Cook high-oxalate foods well — Cooking methods that involve boiling can help reduce oxalate content in foods as the oxalates will leach into the cooking water. |
| Topical calcium for oxalate-related skin irritations — Applying topical calcium can alleviate your symptoms by precipitating oxalates at the site. |
Remember that healing your body takes time — don’t expect results overnight. It’s a marathon, not a sprint. In some cases, it may take two years to two-and-a-half years after following a low-oxalate diet to see the effects, and they may not be pleasant.
For example, you may suddenly get sicker, as your kidneys are finally cleaned up and can excrete oxalate more efficiently. This means your body is tapping into deeper deposits. Possible side effects can include gastritis, migraines, anxiety attacks, gout and other types of toxic reactions.
Your uric acid may also increase, as it is replacing the oxalic acid. In this instance, this means you’re clearing oxalate. You may also notice tartar buildup on your teeth, gritty stools, gritty eyes, hemorrhoids and burning stools — all these are symptoms that your body is healing itself.
Cellular Energy — The Very Essence of Life
My personal struggle with the skin irritation triggered by oxalates 15 years ago is an eye-opener. It’s what I consider the pivotal turning point in my health journey, as it is the best illustration of just how crucial it is to have a healthy, well-functioning microbiome to your overall health.
Unfortunately, virtually none of us have a healthy gut microbiome. This is a result mainly because of large multinational corporations taking advantage of us and steering us toward unnatural products that end up harming our mitochondria and ultimately our ability to create cellular energy.
I believe that your ability to produce sufficient cellular energy is the single most important factor to fuel your body’s innate repair and regeneration processes so it can recover from diseases and any type of health obstacle.
With that said, I will be releasing a new book this summer that delves into the science of cellular energy. In this book, I’ll explain in detail the biochemical pathways that provide energy to your cells, as well as also how disrupting these pathways can put you or your loved ones at risk of progressively worsening health issues.
I’ll also share practical strategies to help support your mitochondrial health and enhance your cellular energy production through healthy food choices, lifestyle changes and proper supplementation. This book is a definite must-read, as it can help you rediscover the foundational strategies to heal your body and ward off diseases, so stay tuned.
The Amazing Benefits of Dairy Fat
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2023/12/26/dairy-fat-benefits.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola December 26, 2023
STORY AT-A-GLANCE
- Studies have repeatedly failed to find an association between full-fat dairy and cardiovascular events. Instead, full-fat dairy actually reduces your risk of cardiovascular events and deaths thereof. Dairy products are also associated with lower risks of Type 2 diabetes, liver disease and more
- Whole-fat dairy contains the odd-chain saturated fats (OCFAs) pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0), which have significant health benefits
- OCFAs are found only in small amounts in certain foods, primarily dairy fat, and your body only makes C17:0. Researchers now believe C15:0 may be an essential fat, as your body cannot make it
- Higher circulating levels of OCFAs in the blood is associated with lower risks of obesity, chronic inflammation, cardiovascular disease, metabolic syndrome, Type 2 diabetes, NASH, COPD, pancreatic cancer and all-cause mortality
- OCFAs do not have an inhibitory effect on glucose burning because they are not converted to acetyl-CoA; rather, they enter the Krebs Cycle as succinyl-CoA. What this means in practical terms is that you don’t need to restrict your consumption of full fat dairy, as it won’t affect your ability to burn glucose
Do you avoid whole milk, or better yet, raw milk, because of its saturated fat content? If so, you may be missing out on one of the greatest health foods there is. Studies1 have repeatedly failed to find an association between full-fat dairy and cardiovascular events. Instead, they’ve found the opposite — full-fat dairy reduces your risk of cardiovascular events and deaths thereof.
Dairy products are also associated with lower risks of Type 2 diabetes,2 liver disease and more. One of the reasons for these health benefits is because whole-fat dairy contains health promoting compounds such as:3
| Specific amino acids | Unsaturated, medium-chain, and branched-chain fats |
| Odd-chain saturated fats — pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) | Phospholipids |
| Vitamins and minerals | Probiotics |
Odd-Chain Saturated Fats From Dairy Are Likely Essential Fats
Of these, the odd-chain saturated fats (OCFAs) are of particular importance. In fact, recent research4 suggests these are likely one of the most essential fats in the human diet, unlike linoleic acid (LA) that most foods are loaded with. It’s virtually impossible to become deficient in LA outside of a laboratory diet.
The same cannot be said for the OCFAs. You need to get them from dairy, because that’s the primary source. As noted in the 2020 scientific report, “Efficacy of Dietary Odd-Chain Saturated Fatty Acid Pentadecanoic Acid Parallels Broad Associated Health Benefits in Humans: Could It Be Essential?”:5
“Dietary odd-chain saturated fatty acids (OCFAs) are present in trace levels in dairy fat and some fish and plants. Higher circulating concentrations of OCFAs, pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0), are associated with lower risks of cardiometabolic diseases, and higher dietary intake of OCFAs is associated with lower mortality.
Population-wide circulating OCFA levels, however, have been declining over recent years. Here, we show C15:0 as an active dietary fatty acid that attenuates inflammation, anemia, dyslipidemia, and fibrosis in vivo, potentially by binding to key metabolic regulators and repairing mitochondrial function.
This is the first demonstration of C15:0’s direct role in attenuating multiple comorbidities using relevant physiological mechanisms at established circulating concentrations.
Pairing our findings with evidence that (1) C15:0 is not readily made endogenously, (2) lower C15:0 dietary intake and blood concentrations are associated with higher mortality and a poorer physiological state, and (3) C15:0 has demonstrated activities and efficacy that parallel associated health benefits in humans, we propose C15:0 as a potential essential fatty acid.”
Dietary Guidelines Got It Backward
The low-fat recommendation has been around for more than 40 years, and since that time cholesterol levels and heart disease rates have gone in the opposite direction of what was intended.
As noted in the featured paper,6 in the two decades following that recommendation, average intake of whole fat milk dropped more than fourfold, from 283 grams to 65 grams a day, yet prevalence of obesity, Type 2 diabetes, metabolic syndrome and nonalcoholic fatty liver disease (NAFLD) rose to new heights.
Meanwhile, researchers kept finding that people who consumed whole fat milk had lower risks of obesity, Type 2 diabetes and cardiovascular diseases. Clearly, something was off. At the same time, consumption of LA skyrocketed as saturated fats were replaced with processed seed oils, and we now have robust evidence showing that excessive LA is a key driver of these chronic diseases, as it destroys mitochondrial function and metabolism.
In short, the U.S. dietary guidelines discouraged consumption of what now appears to be a TRULY essential fat — odd-chained saturated fats found in milk — while encouraging consumption of what was believed to be an essential fat, but in fact is one of the most destructive ingredients in the modern diet.
Contrary to popular belief, LA is not an essential fat, in part because it’s found in most foods, making it near-impossible to become deficient. Odd-chained saturated fats, on the other hand, are found only in small amounts in certain foods, primarily milk, and your body only makes C17:0. It doesn’t appear to make any C15:0 endogenously,7 which means you have to get it from your diet.

Download this Article Before it Disappears
Milk Fats 101
As you can see by the list above, whole milk contains several different kinds of fat. About 68% of the fats are even-chain saturated fats (ECSFs), the primary ones being:8
- Myristic acid (C14:0)
- Palmitic acid (C16:0)
- Stearic acid (C18:0)
The odd-chain saturated fat (OCFA) pentadecanoic acid (C15:0) represents only 1% of the fat content, and heptadecanoic acid (C17:0) makes up 0.5% of the total.9
OCFAs Linked to Lower Disease Risk
Previous studies have shown that higher dietary intake of OCFAs, and subsequently higher circulating levels of OCFAs in the blood, is associated with LOWER risks of:10
| Obesity | Chronic inflammation |
| Cardiovascular disease | Metabolic syndrome |
| Type 2 diabetes | Nonalcoholic steatohepatitis (NASH) |
| Chronic obstructive pulmonary disease (COPD) | Pancreatic cancer |
| All-cause mortality |
In the video above, Dr. Paul Saladino reviews studies showing similar benefits for butter. For example, one eight-week-long randomized controlled trial found people who ate about 1.5 tablespoons of butter per day had lower levels of inflammation (based on inflammatory markers) at the end of the trial.
A Closer Look at OCFAs
To get a better idea of how OCFAs affect human health and prevent disease, the featured Scientific Reports paper conducted a series of in vitro and in vivo studies using 99% pure OCFAs. First, OCFAs were tested for peroxisome proliferator-activated receptor (PPAR) agonist activity.
There are three primary PPARs: α (alpha), δ (delta) and γ (gamma). PPARs are transcription factors known to reduce triglyceride levels when activated.11 They’re also involved in the regulation of metabolism and inflammation, and they do that by detecting and responding to the presence of dietary fats.12
Agonists are compounds that activate a given receptor, so what they were looking for was whether OCFAs might work by activating PPARs.
They also assessed the impact of OCFAs on mitochondrial function and the production of reactive oxygen species (ROS). Mitochondrial dysfunction is at the heart of all disease, and elevated ROS production, which is indicative of inflammation, is another hallmark of most, if not all, disease states.
OCFAs were also tested across a variety of human cell systems mimicking chronic inflammatory and fibrotic disease states. Next, the effects of oral OCFA supplementation were studied in in vivo models of cardiometabolic, inflammatory, liver, hematologic, and fibrotic diseases. Here’s a summary of what they found:13
| C15:0 is a dual, partial agonist for PPARα (65.8%) and PPARδ (52.8%). Effective concentrations of C15:0 needed to reach half-maximum activities for PPARα and PPARδ were 11.5 and 2.7 micrometer (µM), respectively. |
| C15:0 repaired mitochondrial function and reduced mitochondrial ROS production in a dose-response u-curve. Mitochondrial function and reduced ROS were found in cells supplemented at doses of 10 µM, 20 µM and 50 µM, but at C15:0 concentrations of 100 µM and 200 µM, there were no differences in ROS production compared to non-supplemented controls. |
| C15:0 reduced proinflammatory and profibrotic states in the human cell systems tested. C17:0 also had these effects, but to a lesser degree.
According to the authors, “this study … supports that a relatively minor increase in C15:0 concentrations (e.g. from 2.2 µM to 6.7 µM) can positively impact its anti-inflammatory and antifibrotic activities.” On a side note, µM is a unit of measure, not a dose. To convert µM to milligrams (mg), divide it by 1,000. So, a concentration of 2.2 µM equates to 0.0022 mg and 6.7 µM is 0.0067 mg. |
| Daily supplementation of C15:0 at a dose of 5 mg per kilo of bodyweight lowered inflammation, glucose and cholesterol levels in obese mice. |
| Daily supplementation of C15:0 at a dose of 35 mg per kilo of bodyweight improved hemolytic anemia in rabbits with diet-induced hypercholesterolemia, anemia and NASH, decreasing the loss of red blood cells and lowering new red blood cell production.
This dose also resulted in lower cholesterol, triglycerides, globulins, and platelets compared to non-supplemented diseased controls. Liver health indices also improved to the point they matched that of healthy controls. They had less severe liver fibrosis, and unlike the diseased controls, they did not progress from Stage 2 to Stage 3 fibrosis. |
| C15:0 had no off-target pharmacological activities and was noncytotoxic across the 12 human cell systems tested. |
| C17:0 is a PARδ agonist, with a maximum activity of 39.8%. To achieve half-maximum PPARδ activity, a concentration of 17.4 µM was required. |
| The ECSFs myristic acid (C14:0) and palmitic acid (C16:0) had similar activity as C15:0. Both are agonists for PPARα and PPARδ, leading the researchers to hypothesize that “carbon chain length may be a determinant of PPARα/δ binding.” |
| None of the saturated fatty acids had PPARγ agonist activity at concentrations below 100 µM. |
What Dose of OCFA Will Achieve These Benefits?
To assess the dose required to achieve these kinds of benefits, they gave an oral dose of C15:0 at 35 mg per kilo of body weight to Sprague Dawley rats. Within 30 minutes, plasma concentrations of C15:0 were increased. Maximum concentration (20 µM) was achieved at one hour post-ingestion, and plasma levels remained above baseline for 24 hours.
“Thus, a single oral dose of C15:0 at 35 mg/kg succeeded in achieving our targeted active plasma concentrations in this rodent model, between 2.5 to 5 µg/ml (equivalent to 6.7 to 20 µM), from 1 to 8 hours post-dose,” the authors write.14 “Plasma total C17:0 levels also increased, albeit less so than C15:0, following a single oral dose of C15:0.”
To further evaluate the safety of C15:0, rats were dosed orally once a day for 14 days with increasing doses, up to 350 mg per kilo of body weight. No abnormalities were found, and there were also no significant differences in body weights, organ weight-to-body weight ratios, abnormal chemistry values or histologic observations between those who got the C15:0 and the controls.
C15:0 Is Likely an Essential Fat
Based on the findings from this investigation, the researchers concluded that C15:0 (but not C17:0) is most likely an essential fatty acid:15
“Essential fatty acids are defined as active dietary fatty acids that: (1) are required to maintain a healthy physiological state, (2) are not made at adequate levels endogenously, and (3) require dietary intake in order to maintain healthy concentrations in the body.
Given our demonstration of C15:0 and C17:0 as active dietary fatty acids, we reviewed the literature for evidence supporting or negating C15:0 and C17:0 as potential essential fatty acids.
Due to reported direct correlations between dietary C15:0 intake and circulating C15:0 concentrations, indicative of primarily diet-based drivers of circulating C15:0, and evidence of endogenous production of C17:0, only C15:0 had supportive evidence across all three criteria that were consistent with a potential essential fatty acid …
Chronic low-grade inflammation, driven by proinflammatory chemokines and cytokines, contributes to cardiometabolic comorbidities and the aging process.
Here, daily oral supplementation with C15:0 and C17:0 lowered proinflammatory states in obese mice with metabolic syndrome, as well as lowered proinflammatory biomarkers in primary human cell systems mimicking chronic inflammation …
Dyslipidemia and hyperglycemia are components of metabolic syndrome, a cluster of conditions impacting approximately 1 in 3 people globally. Metabolic syndrome increases the risk of type 2 diabetes, heart disease, and all-cause mortality.
In our studies, daily oral C15:0 supplementation over 12 weeks lowered total cholesterol and glucose in an in vivo model with metabolic syndrome.”
Dairy Fat — It Does Your Body Good
The take-home from all of this is that diary fat is a crucial source of an essential fat — pentadecanoic acid or C15:0 — that your body needs and cannot make.
A long list of studies16,17 through the years have shown that this and other OCSFs improve mitochondrial function and increase ATP production,18 lower your risk of obesity,19 diabetes20,21,22 and cardiovascular disease,23 including the risk of heart problems in diabetics,24 promote healthy hair growth,25,26 lower inflammation and much more.
While you can drink whole fat milk, it has 4% fat. Butter has 20 times the fat concentration of whole fat milk, and ghee 25 times the fat as whole fat milk. It is far easier to get these odd chain saturated fats by eating a healthy butter. Ghee is easer and has 25% more fat than butter.
A reasonable dose for most people is 1 tablespoon of butter a day. You can increase that, but it would be unwise to go over 5 tablespoons a day. Unlike raw milk, high quality butters are far more difficult to purchase commercially. About the only way you can is to purchase directly from the farmer.
If you are looking for an easier commercial solution, you can purchase 1 pound of organic ghee for about $20 a pound, including shipping.
Milk Fats Do Not Inhibit Glucose Metabolism
Interestingly, OCFAs are only partially metabolized via the beta-oxidation pathway that ECFAs use. In this pathway, fats are first converted to acetyl-CoA, which allows them to enter the Krebs Cycle. OCFAs, in contrast, are first converted into succinic acid, then succinyl-CoA, which then enters the Krebs Cycle and helps support electron transfer at complex II in the mitochondria. As explained by Georgi Dinkov, an expert on bioenergetic medicine:27
“Since rising levels of acetyl-CoA has an inhibitory effects on pyruvate dehydrogenase (PDH), eating a diet high in fat with mostly even-chain fats would result in reduction of glucose metabolism, even if all the fats are of the SFA type, as per the Randle Cycle.
However, if those fats are of the odd-chain species and enter the Krebs Cycle as succinic acid (i.e. without effect on the acetyl-CoA/CoA ratio), then virtually no such reduction of glucose metabolism is expected to occur and, in fact, PA [pentadecanoic acid] was described in … Japanese studies as stimulating mitochondrial function and ATP production, which ultimately resulted in improved hair growth.
The Japanese researchers even filed a patent for treating hair-loss with PA and in that patent they opined that other odd-chain fatty acids with similar length, especially the C17:0 fat … would have similarly beneficial effects on hair-growth through increasing mitochondrial function (OXPHOS).”
Put another way, as I’ve described in several previous articles, including “Crucial Facts About Your Metabolism,” when your fat intake is too high (likely above 35 grams or so), then your body’s ability to metabolize (burn) glucose in your mitochondria is reduced. Instead, it gets shuttled into the glycolysis pathway, which is extremely inefficient and produces far fewer ATP molecules per molecule of glucose. That’s what Dinkov is talking about here.
What’s fascinating is that OCFAs do not have this inhibiting effect on glucose burning, because they are not converted to acetyl-CoA but rather enter the Krebs Cycle as succinyl-CoA. What this means in practical terms is that you don’t need to restrict your consumption of full fat dairy, as it won’t affect your ability to burn glucose.
Equally interesting, excessive consumption of other dietary fats has been shown to lower your plasma level of C15:0 and C17:0.28 So, there are more reasons than one to make sure your total fat intake isn’t too high.
- 1 The Lancet November 24, 2018; 392(10161): 2288-2297, DOI: 10.1016/S0140-6736(18)31812-9, Page 2
- 2 PLOS Medicine October 10, 2018, DOI: 10.1371/journal.pmed.1002670
- 3 The Lancet November 24, 2018; 392(10161): 2288-2297
- 4, 5, 6, 8, 9, 10 Scientific Reports 2020; 10: 8161
- 7 Scientific Reports March 23, 2017; 7: Article number 44845
- 11 J Adv Pharm Technol Res October-December 2011; 2(4): 236-240
- 12, 13 Scientific Reports 2020; 10: 8161, Results
- 14 Scientific Reports 2020; 10: 8161, Oral C15:0 achieved active concentrations in vivo
- 15 Scientific Reports 2020; 10: 8161, There is supportive evidence of C15:0 as a potential essential fatty acid
- 16, 23, 27 Haidut.me December 5, 2023
- 17 Scientific Reports 2020; 10: 8161, See reference list
- 18, 25 International Journal of Cosmetic Science June 1993; 15(3): 125-131
- 19 Pediatric Obesity May 2020; 15(5):e12612
- 20 Med J Islam Repub Iran December 18, 2017; 31: 110
- 21 NPR April 18, 2016
- 22 Clinical Nutrition June 18, 2021; 40(8): 4988-4999
- 24 Deseret News July 14, 2023
- 26 J-Stage 1995; 37(6): 800-806
- 28 The International Journal of Biochemistry & Cell Biology February 2022; 143: 106135
Schizophrenia Is Chronic Encephalitis …and Niacin Cures It
Reproduced from original OMNS article (OrthoMolecular News Service):
http://orthomolecular.org/
Subscribe to the free Orthomolecular Newsletter: http://orthomolecular.org/subscribe.html
Go to the OMNS Archive: http://orthomolecular.org/resources/omns/index.shtml
Orthomolecular Medicine News Service, October 12, 2023
by Thomas E. Levy, MD
OMNS (October 12, 2023) Orthomolecular medicine is based on the concept that most chronic diseases are ultimately initiated, and then sustained, by the chronic deficiency of one or more vitamins, minerals, nutrients, or other natural agents. When the deficiency can be lessened, the disease improves. Conversely, the worse the deficiency and the longer it persists in the body, the more advanced and entrenched the disease becomes. What often is unclear for both the public as well as many healthcare providers is that the clinical benefits of some nutrient supplements continue to increase as the doses are increased. These doses can vastly exceed the Recommended Dietary [or Daily] Allowance (RDA) disseminated by the Food and Nutrition Board, a committee established by the United States National Academy of Sciences. Since 1997, the term Dietary Reference Intake (DRI) has been in use to describe much the same information as the RDA. The DRI recommendations have not significantly deviated from the earlier RDA recommendations. [1]
While a few nutrients can rapidly become toxic with minimally excessive intake (calcium, copper, and iron), many nutrients have little toxicity at almost any dose. [2] In general, the doses of vitamins are difficult to push to the point of clinical toxicity. However, nearly all the nutrient minerals can readily be taken to excess and result in various presentations of toxicity. Toxicity in this context refers to definable physiological damage to the supplement taker, not occasional side effects such as nausea in a sensitive stomach (niacin) or osmotic diarrhea (vitamin C or magnesium) when too much is not efficiently absorbed but accumulates in the colon instead.
However, the concern about potential toxicity keeps supplements like niacin, vitamin C, and magnesium severely underdosed, resulting in a loss of the incredible benefits they offer when optimally dosed.
Vitamin C and Magnesium Supplementation
Vitamin C is the safest of all known nutrient supplements. In fact, there has never been established any dose of vitamin C above which toxicity will reliably ensue. This is consistent with the fact that vitamin C is the molecule on which the physiology of all cells runs, and the healthy function of the body relies on having large amounts of it both inside the cells as well as outside of them. Arguably, vitamin C is the safest consumable agent in existence. Rare individuals can experience minimal side effects, but this should not be confused with any degree of cell-damaging toxicity. By contrast, too much water intake is toxic and can even result in death. [3-5]
The vitamin C RDA for older children and adults ranges from 45 to 90 mg per day. However, many people maintain a much higher level of general health when multigram supplementation is taken regularly, on the order of 100 times the RDA. Furthermore, the administration of vitamin C in doses 1,000 times the RDA are frequently given intravenously around the world for the treatment of a wide range of infections and medical conditions, with excellent effect and unrivaled safety. [6-8]
Magnesium, like all minerals, can be pushed to toxic levels of intake. However, it is almost impossible to induce toxicity with ORAL magnesium intake, as the highest levels of intake will reliably induce an osmotic diarrhea from unabsorbed magnesium reaching the colon. But when given intravenously, enough magnesium will reliably lower even the most elevated of blood pressures to hypotensive levels. In some surgeries, sufficient magnesium is infused to deliberately keep blood pressure below normal levels to help achieve hemostasis and keep the surgical field from bleeding excessively. [9-11]
Such highly-dosed infusions should only be administered in a hospitalized setting. However, the addition of a few grams of magnesium can always be added to a therapeutic vitamin/mineral IV bag and be safely infused over an hour or so in the clinic setting. In fact, the appropriate administration of magnesium by IV infusion is the best way to help restore low body levels of magnesium, especially in patients who cannot take very much orally. [12,13] Some caution and dosage adjustments need to be made by the clinician when there is decreased kidney function.
Just like vitamin C, but much less dramatically so, oral magnesium supplementation of several grams daily can be taken as long as the osmotic diarrhea is not induced. With a magnesium RDA of roughly 300 to 400 mg daily, the amounts of supplementation to keep most adults out of a significant deficiency of magnesium will be in the range of 5-fold or more of this RDA. Furthermore, very few people can reach an optimal magnesium status with oral supplementation. Rather, the practical goal is to minimize the degree of magnesium deficiency. Nevertheless, as a significant magnesium deficiency causes some diseases and makes all diseases worse, taking as much magnesium supplementation as can be readily tolerated is always a good idea. [14]
Of the 13 essential vitamins (A, C, D, E, K, B1, B2, B3, B5, B6, B7, B9, B12), increased intake and/or increased blood levels have been associated with decreased all-cause mortality for 11 of them. [15-23] Studies clearly establishing the same associations with biotin (vitamin B7) and pantothenic acid (vitamin B5) were not found. For the most part, these studies only examined vitamin intakes in the range of the RDA or DRI values, further supporting their critical support of good health even when ingested in relatively small amounts. While toxic effects can be seen with vigorous dosing of vitamin A, vitamin D, or vitamin E, the rest have RDA or DRI values that can be greatly exceeded, resulting in only improved health and blood chemistries.
Niacin: Nomenclature and Physiology
Confusion can easily arise in sorting through the literature on niacin and its derivatives. Niacin is vitamin B3. It is also known as nicotinic acid. These are all synonyms for the chemically identical substance. Niacin, vitamin B3, and nicotinic acid are completely interchangeable terms. For completeness, niacin is also rarely referred to in the literature as “vitamin PP,” with the PP meaning “pellagra-preventive.” Pellagra is the clinical condition that results from a severe deficiency of niacin in the body. [24]
Niacin has several vitamers. Vitamers are derivatives or related chemical substances that fulfill the same specific vitamin functions despite not being chemically identical. Niacin derivatives that qualify as vitamers include niacinamide (also known as nicotinamide or nicotinic acid amide), nicotinamide riboside, and nicotinamide mononucleotide. Referring to nicotinamide as niacinamide decreases the possibility of niacin and its vitamers as being perceived by the public as having nicotine-like properties, which it does not. All these substances promote the biosynthesis of NAD (nicotinamide adenine dinucleotide) throughout the body, and they are the primary sources of NAD. [25,26]
Large amounts of NAD are essential for optimizing the electron supply in the first of the four steps of the electron transport chain (ETC). Located along the membranes of the mitochondria inside every cell, the ETC is responsible for the production of all the ATP (adenosine triphosphate) in the body. ATP is the most important energy-providing molecule in the body. Any compromises in its production results in a decline in the healthy function of all affected tissues and organs. When there is not enough NAD present at the beginning of the ETC, sufficient ATP simply cannot be generated.
Optimizing the production of NAD for ATP synthesis in the cells is the most important function of niacin and its vitamers.
Furthermore, greater deficiencies in available NAD results in even more pronounced declines in cellular function throughout the body. Nothing is more important for optimal health than maximal amounts of intracellular ATP. [27] Low NAD levels have been recognized as a sign of aging in not only humans, but in all living cells, including those in animals and insects. [28-34]
Niacin Supplementation
Names of forms of niacin supplementation that directly fuel NAD production in the body:
- Niacin
- Niacinamide
- Nicotinamide
- Nicotinamide riboside
- Nicotinamide mononucleotide
- Inositol hexaniacinate
- Inositol hexanicotinate
Of note, niacin has an additional important property that its vitamers do not have. Reported as early as 1955, niacin has been documented to lessen the abnormal lipid metabolism that promotes atherosclerosis. [35-37] It reduces triglycerides and the lipoproteins VLDL and LDL while raising HDL, the “good” lipoprotein. [38]
If well-tolerated, niacin is the best of the supplement forms itemized above to take, as it has both the positive impact on the lipids as well as on the NAD levels in the body. It also costs less. However, niacin causes a warm to hot flushing effect in many people who supplement it. While for many people this flushing effect is either minimal or even disappears after several doses, for some people it is not tolerable. The other supplement forms noted above are largely “flush-free,” and can be easily taken by nearly everyone. The downside is that the non-flushing forms do not have the positive lipid impact of unmodified niacin.
Niacin and all its vitamers profoundly impact ATP generation throughout the body, as noted above. However, like so many other powerful orthomolecular therapies, the niacin RDA and DRI is amazingly tiny, completely misleading the health seeker as to its importance and impact of much higher doses. The optimal energy-supporting doses of niacin can be 200- to 1,000-fold higher than these officially-advised doses. And other than nausea in a few individuals, side effects are decidedly uncommon. [39] Very high doses have been linked to liver toxicity, as reflected in significant liver enzyme elevation. However, minor enzyme elevation that typically resolves without discontinuation of the supplementation is not uncommon. Such enzyme increases are felt to represent a temporary increased metabolic activity in the liver cells and not inflammatory damage. [40]
In the toxin-laden, pro-oxidant environment in which we now all live, virtually everyone is deficient in the antioxidant impact provided by niacin supplementation and the NAD levels it supports. Everybody should take at least some niacin supplementation. There really does not exist a dietary regimen that can provide the NAD-producing benefits of even a minimal supplementation of niacin.
Niacin, Health, and Schizophrenia
Optimizing the production of ATP in the body is a very desirable goal. Many clinicians today regard chronic fatigue patients as having “mitochondrial dysfunction” or “mitochondrial fatigue.” While decreased ATP production is uniformly present in such patients, different patients can have different reasons for that decline in production. [41] However, except for individuals with genetic deficiencies that typically cannot be completely resolved, increasing the production of ATP not only can resolve the fatigue and associated symptoms, it can also infuse the needed energy into the dysfunctional metabolic pathways to completely resolve the biochemical abnormalities that decreased the ATP production in the first place. Quite literally, this results in cellular healing. Niacin supplementation has been shown to restore healthy NAD levels (which then increase ATP production), greatly improving muscular strength in patients with mitochondrial dysfunction. [42]
Ultimately, all such dysfunction inside the cytoplasm as well as inside the mitochondria comes from increased numbers of inactivated, oxidized biomolecules relative to the numbers of normal, reduced biomolecules. This is traditionally referred to simply as increased oxidative stress. Improvements in all pathological states can be anticipated with increased ATP production, although certain conditions, such as muscle fatigue from low ATP levels, can be expected to respond even more dramatically. The heart muscle in heart failure is a classic example of a tissue severely depleted of ATP, no longer able to respond to significant exercise with a sufficiently increased production of ATP. [43]
Endomyocardial biopsies have documented that heart muscle in congestive and hypertrophic cardiomyopathies have significantly depressed levels of both ATP and NAD. [44] Normal heart muscle has the highest NAD levels in the body. [45] In both congestive and hypertrophic cardiomyopathy impaired energy metabolism has been identified. [46] Consistent with these findings, the elevation of NAD levels in different studies has been shown to improve atherosclerosis as well as different forms of heart failure, including ischemic, hypertrophic, and congestive cardiomyopathies. [47,48] In an animal study, niacin has also been shown to lessen damage in myocardial infarction. [49] Studies in both animals and humans have shown that niacinamide can lower elevated blood pressures and decrease cardiac mortality. [50,51] In a mouse model of cardiac arrest, niacinamide administration was able to normalize NAD levels and improve survival. [52]
Niacin vitamer supplementation in humans has been clearly shown to dramatically increase blood levels of NAD. [53] Not surprisingly, NAD-increasing agents, with their strong support of ATP production, are also being increasingly appreciated as being useful for both anti-aging and overall good health. [54-59] In an animal study of sepsis, probably the most advanced and dire of medical conditions, a niacin vitamer was shown to increase survival and prevent the lung and heart injury otherwise seen. [60]
Some studies indicate that lower NAD and ATP levels are the primary abnormalities that result in cancer. [61-63] In one human study, it was shown the niacinamide supplementation was effective in reducing the appearance of new skin cancers. [64] This is consistent with the pellagra-associated skin inflammation (dermatitis) seen when niacin levels are very low. [65] Higher niacin intake has been linked to decreased all-cause mortality, indicating its importance in every cell in the body. [66,67]
The clinical effectiveness of daily multigram niacinamide dosing depends on how severely the affected tissues or organs in a disease are depleted of the NAD needed to make ATP. Heart failure, while not always responsive to increased NAD production, will often respond dramatically to an improved NAD status. A significant number of patients with congestive cardiomyopathy and low cardiac output have been spared heart transplantation after adequately-dosed Coenzyme Q10 (CoQ10), another agent capable of increasing ATP production via the ETC in the mitochondria. In many of those patients, ejection fractions have increased dramatically, and all-cause mortality decreased along with an improved exercise capacity. [68-74] Also, like niacinamide, CoQ10 also improves heart failure patients with preserved ejection fractions (hypertrophic cardiomyopathy with diastolic dysfunction). [75,76]
Niacinamide has also been shown to enhance acetyl-CoA production, which in turn enhances the biosynthesis of CoQ10.
This means that niacinamide supplementation can provide the substrates needed for both NAD and CoQ10 production, directly powering two of the four steps of the ATP-producing ETC. [77,78]
Like heart muscle, the brain and central nervous system (CNS) require very high levels of ATP for normal function relative to the rest of the body. As such, having inadequate building blocks for ATP production in the body will be reflected more often and more prominently as nervous system and psychiatric disorders than other medical conditions. Many studies have indicated that niacinamide is essential for the development, growth, and maintenance of the CNS. [79-81] Furthermore, niacinamide has been shown to readily pass the blood-brain barrier in both directions, supporting its supplementation as a simple and effective approach to treating various CNS conditions. [82] Also, oral niacinamide supplementation has been shown to be very well-absorbed. [83]
Animal studies have shown that niacinamide will protect against ischemia-induced (decreased blood supply) damage in the brain and CNS. Neuronal death is reduced, and the recovery of affected sensory and motor function is improved as well. [84-87]
Significant infection protection and resolution can be seen when NAD production is optimized with niacin and several other NAD-producing agents. Sepsis recovery is supported by increased niacin levels. COVID has also been shown to resolve more rapidly with agents that help to optimize cellular ATP levels. [88-91] Improved outcomes in COVID-related acute kidney injury have been seen with niacinamide therapy. [92] Niacinamide has also been shown to lessen inflammation-induced renal failure in an animal model. [93] It has a protective effect against the pro-inflammatory toxicity of paraquat in rats. [94]
Severe niacin deficiency results in a disease known as pellagra. This syndrome has been characterized by a triad of symptoms: delirium, dermatitis, and diarrhea. More accurately, the triad should be referred to more broadly as neurocognitive, dermatological, and gastrointestinal symptoms. A substantial variation in this symptom pattern can be seen, as the circumstances resulting in a deficiency of niacin can result in a variety of other significant nutrient and micronutrient deficiencies in a given patient. [95-97] Nevertheless, the complete restoration of niacin levels in the body, along with the micronutrients supplied by a balanced diet, reliably resolves the symptoms of pellagra, including those involving the CNS.
The neurocognitive symptoms in patients with severe niacin deficiency are always present, and they can be very pronounced clinically. Both Alzheimer’s disease and Parkinson’s disease typically have depleted NAD levels in the affected tissue, and some of their symptoms can be lessened with increased niacin intake. [98] Other CNS symptoms, including disorientation, memory loss, confusion, dementia, poor sleep, and even frank psychosis can be seen in the severely niacin-deficient patient. [99] Of note, the statistical risk of Parkinson’s disease is lessened in individuals having an increased consumption of niacin-containing foods. [100,101]
Schizophrenia is one of the most devastating of diseases, with enormous societal impact in addition to the symptoms endured along with the effective loss of a functional life in the patient. [102] Chronically increased oxidative stress defines a state of chronic inflammation in the brain (neuroinflammation). The presence of chronic inflammation in the brains of schizophrenic patients has been well-documented. [103,104] Studies have shown that schizophrenia in younger individuals can be initiated following exposure to the toxins, or pro-oxidants, encountered in prenatal exposures to infection. [105,106] Consistent with this, many cases of schizophrenia start early in life with toxin-induced abnormal neurodevelopment. [107,108]
The symptoms of schizophrenia are numerous and diverse, with some symptoms assuming a much more prominent role in one patient versus another. The bulk of the literature simply recounts the classical and well-known symptoms of schizophrenia that focus solely on brain dysfunction. Such symptoms include hallucinations, delusions with loss of contact with reality, difficulty thinking clearly, and social/emotional withdrawal, sometimes to the point of staying in a largely motionless, catatonic state. However, it has also been recognized that symptoms not directly referable to brain dysfunction are often present as well. These include the classical symptoms of pellagra, the potentially fatal condition secondary to severe niacin deficiency in the body.
The niacin deficiency in pellagra can result brain and CNS pathology manifesting as irritability, difficulty concentrating, some social withdrawal, depression and manic depression, insomnia, delirium, hallucinations, coma and even frank psychosis. Some authors have termed this “pellagroid encephalopathy.” [109] Furthermore, the psychosis with associated delusions that sometimes occurs in pellagra is indistinguishable from some cases of schizophrenia. [110] These symptoms all typically resolve with adequate restoration, and maintenance, of niacin levels in the body. [111] The effective use of niacin for psychiatric symptoms has also spawned the concept of “reversible dementia,” a remarkable term since dementia is generally considered to be progressive and non-resolving in nature, especially in older individuals. [112] Similarly, the close relationship between pellagra and brain dysfunction has spawned the term “niacin-respondent subset of schizophrenia.” [113]
The niacin deficiency in pellagra always has symptoms of brain dysfunction, and niacin restoration treats them very effectively.
Pellagra causes significant gastrointestinal problems and symptoms. This is significant in understanding the contribution and worsening that a pellagra-related leaky gut with a pathogen-overgrown microbiome does to diseases of the CNS (and elsewhere in the body). Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis (ALS), and schizophrenia have all been clearly documented to either have pathogens and/or their toxic metabolites present in the affected nervous tissue. [114-125] The damage that niacin deficiency (pellagra) does to the microbiome is a major factor in the associated neuropsychiatric problems and schizophrenia-like symptoms seen with it as well. Niacin deficiency not only results in decreased energy production in the brains of schizophrenics, it also results in the continued exposure of pathogens and/or their toxic metabolites from an abnormal gut microbiome to the CNS of those patients.
Schizophrenia most commonly appears in late adolescence or early adulthood. [126] But it can occur later in life as well. Increased intracellular oxidative stress, left unchecked, results in premature death of the affected cells. Brain volume studies have established that schizophrenic patients progressively lose gray matter and the actual physical mass of the brain over time, well beyond the deterioration seen with aging. [127-130] Microglia, the scavenger white cells of the brain, become activated in the inflammation seen in schizophrenia. [131-133] Pro-inflammatory cytokines and other inflammatory markers are also increased. [134-136] All this information leads to the following assertion:
Schizophrenia is chronic encephalitis.
Encephalitis is inflammation of the brain, typically occurring acutely in conjunction with a new viral infection precipitating widespread inflammation throughout the brain and CNS. In schizophrenia the chronically elevated inflammatory parameters indicate that ongoing inflammation is causing the signs and symptoms of schizophrenia. Schizophrenia = chronic brain inflammation = chronic encephalitis. [137] Many of the symptoms seen in acute encephalitis brain are also seen in the chronic encephalitis brain of the schizophrenic patient, including alterations in consciousness, confusion, hallucinations, and cognitive impairment. Some authors have descriptively referred to schizophrenia as “the shattered mind.” [138] The steady destruction of brain tissue by the chronic inflammation also explains why schizophrenia present for years responds less readily to any nutrient or drug regimen than schizophrenia of recent onset. The chronic inflammation slowly destroying brain tissue in schizophrenia is analogous to the patient with ongoing myocarditis and death of heart muscle eventually going into congestive heart failure. The later a positive therapy is started, the less effective it will be.
The effective clinical resolution of many schizophrenics is further complicated and even impaired by the common and severe side effects seen with the drugs commonly used in its treatment. Many of these side effects are indistinguishable from many of the symptoms for which the drugs are being given. Such symptoms include restlessness, brain fog, and social withdrawal with the loss of desire to interact with others. [139] Once a schizophrenic patient has received prescription drugs for a long enough period, it can become impossible to know when the condition itself is getting worse or a drug needs to be discontinued and/or decreased in dosage. As it is, the clinical picture of an unmedicated schizophrenic patient also spans a variety of symptoms present in many different combinations. [140]
The levels of niacin in schizophrenic patients are always low, oftentimes severely so. This also means that their cellular ATP levels are significantly depressed as well. It has been clearly shown that high doses (relative to RDA or DRI recommendations) of niacin or a niacin vitamer often completely resolves schizophrenia, even in its advanced stages. And when clinical resolution is not complete, significant improvement in the major symptoms of schizophrenia is nearly always seen.
In a group of 30 acute schizophrenia patients, one gram three times daily of niacin or niacinamide were given for only 30 days, and the patients were then followed for one year. 80% of the niacin-treated group recovered versus 33% of the placebo-treated group. [141] Recovery in acute or chronic schizophrenia was only considered to have been reached when the patient
- Had complete disappearance of disease-related symptoms and signs
- Was interacting normally with family members as well as members of the community
- Became gainfully employed
Vitamin C, a perfect treatment for any condition involving chronic inflammation, was often given in a dose of 1 to 10 grams daily as well. As the primary antioxidant (anti-inflammatory agent) in the body, vitamin C should always be used to help resolve the brain inflammation of schizophrenia. [142] Much higher doses will always help, and sometimes dramatically so, especially in schizophrenia of recent onset.
While not typical, acute schizophrenia can spontaneously resolve. Presumably, the factors provoking the inflammation in the brain of those patients eventually resolve, or become much less pronounced (e.g., infection, toxin, autoimmune reaction, micronutrient depletion).
Six more double-blind, randomized and controlled clinical trials confirmed the positive impact of niacin on the recovery of schizophrenic patients. [143,144] Many of the most chronic patients (with the most structural brain damage) required this therapy for five or more years to derive clear benefits. [145] For the niacin treatment of schizophrenia, the starting dose was 1,000 mg three times daily, with the dose slowly increased to as much as 4,500 to 18,000 mg daily, depending on clinical response. For those treated with niacinamide rather than niacin, the daily dose rarely exceeded 6,000 mg due to the increased problems with nausea and stomach sensitivity. [146-148]
Dr. Abram Hoffer treated over 5,000 schizophrenic patients with this niacin protocol. No deaths ever resulted from the administration of niacin. Furthermore, consistent with the wide-ranging positive effects of niacin on NAD levels throughout the body described above, Hoffer noted improvements in many symptoms not directly attributable to schizophrenia in his niacin-treated patients. [149] He also developed a more comprehensive orthomolecular approach to schizophrenia over the course of his years in clinical practice. [150] Currently, over 85% of chronic schizophrenic patients treated with traditional measures never resolve, even if some treatment benefits are realized. Instead, they remain sick and dysfunctional for the rest of their lives when niacin is not at least a part of their treatment program.
The following can be definitively asserted:
Niacin cures acute schizophrenia most of the time. And substantial clinical improvement is the rule even when a complete cure is not realized in acute or long-standing schizophrenia.
As covered above, schizophrenia, with its close connection to pellagra, is a condition precipitated and worsened by multiple factors. A quality diet and a wide array of vitamin and mineral nutrients are mandatory for an optimal clinical response in all these patients. Several reasons account for the varied (but positive) clinical responses of schizophrenia patients treated with niacin. [151] Nevertheless, monotherapy with niacinamide has completely resolved schizophrenia. [152] As an important and non-toxic nutrient vitamin, niacin should NEVER be denied to any patient with any brain disorder, much less schizophrenia. As Dr. Hoffer put it: “Apparently the worst sin in orthodox medicine is to see a recovery for the wrong reason.”
The production of ATP, the final physiological goal in the severely NAD-depleted brain (and body) of the schizophrenic patients is specifically nurtured and supported not only by niacin, but also by riboflavin, CoQ10, and methylene blue. These four agents directly power the different steps in the mitochondrial ETC needed to optimize ATP production, which directly accounts for all healing and good health. And when permanent brain damage is minimal and the symptoms are due to ongoing neuroinflammation, an excellent clinical response can be anticipated, even if a complete cure is not realized. Niacin, CoQ10, and riboflavin comprise a nutrient triad that has been shown to benefit the antioxidant status of breast cancer patients. [153-155] And even though vitamin B3 is important to everyone, its optimal dosing is achieved by few individuals. While it is literally good for everyone, it nevertheless needs to be clearly asserted that:
Everyone with any psychological or psychiatric condition should take niacin or one of its vitamers, and the dose should be maximized before considering such a condition permanent and/or unresponsive.
Currently, the standard of care in psychiatry does not include the routine administration of niacin or niacinamide for schizophrenia or any other mental or emotional disorder.
While the established standard of practice is usually sufficient to protect a physician from malpractice, deliberately avoiding the usage of niacin for schizophrenia after being exposed to much of the literature and information cited in this article nevertheless constitutes clear medical malpractice, even if it remains unadjudicated.
Healthcare practitioners have an obligation, albeit rarely-honored, to stay informed on the science of old, current, and new therapies. The benefit of niacin therapy in schizophrenia and most brain disorders is certainly not a new discovery. As with all other conditions that have been shown to clearly benefit from an orthomolecular approach to addressing vitamin, mineral, and other nutrient deficiencies, a healthcare practitioner should always be open to all legitimate scientific information that the patient might offer. If such a practitioner refuses to even review such information, and/or will not even discuss such information with the patient, it is time to find a new one.
You do not need a doctor to give you niacin, and there are no absolute contraindications to taking it. You can take it for yourself, and you can advise any friend or family member with a neurological or psychiatric condition that you have information indicating that it is often beneficial regardless of the precise diagnosis.
Recap
Niacin and its related compounds have a long history of improving the mental status of a wide variety of mental and emotional disorders. It has been shown to cure or greatly improve most cases of schizophrenia for which it is properly-dosed. These disorders are primarily caused by a severe deficiency of NAD in the ATP-generating mitochondria in all cells. The primary role of niacin is to increase NAD levels, resulting in an improved or normalized amount of cellular ATP, the most important energy-providing molecule in the body. While many other nutrients will be of benefit in schizophrenia, highly-dosed vitamin C and magnesium should always be given to further control and quell the ongoing neuroinflammation.
Pellagra, the disease established to occur after severe and long-standing niacin deficiency, typically presents with significant brain dysfunction, sometimes clinically identical to schizophrenia. Niacin administration often completely resolves such states of psychosis, further supporting the concept that NAD repletion leading to optimal ATP levels in the brain is the root cause of schizophrenia.
A chronic NAD deficiency always causes increased oxidative stress in the affected tissue. This means that schizophrenia is a chronic encephalitis, as chronic encephalitis simply means an ongoing state of neuroinflammation in the brain.
The variability in clinical response of schizophrenia to niacin therapy is primarily due to how many other nutrient deficiencies are present and whether they are properly restored. How long the schizophrenia has been present and how much irreversible brain damage (decreased brain mass) has occurred is also critical in determining how much clinical benefit is realized.
Even though the psychiatric standard of care does not include niacin therapy for schizophrenia, it can only be considered malpractice not to apply it, especially considering the enormous physical, mental, and societal impact of this dreaded disease. Opting not to use a toxic therapy that might only help a little for a relatively minor condition does not apply to niacin for schizophrenia. That reasoning is reserved for many pharmaceutical agents, not natural nutrients.
Dedicated to the work of Abram Hoffer, MD, PhD
(Dr. Thomas E Levy, an OMNS Contributing Editor, is a cardiologist, attorney, and author of 13 books. He can be reached at televymd@yahoo.com. A collection of all his OMNS articles can be easily accessed with the following link under the subheading of “Orthomolecular”: https://www.tomlevymd.com/health_ebytes.php )
References
1. Institute of Medicine (US) Committee on Use of Dietary Reference Intakes in Nutrition Labeling (2003) Dietary Reference Intakes: Guiding Principles for Nutrition Labeling and Fortification. PMID: https://pubmed.ncbi.nlm.nih.gov/24967483/
2. Levy T (2023) http://orthomolecular.org/resources/omns/v19n36.shtml
3. Ferrier I (1985) Water intoxication in patients with psychiatric illness. British Medical Journal (Clinical Research Ed.) 291:1594-1596. PMID: https://pubmed.ncbi.nlm.nih.gov/3935199/
4. de Leon J, Verghese C, Tracy J et al. (1994) Polydipsia and water intoxication in psychiatric patients: a review of the epidemiological literature. Biological Psychiatry 35:408-419. PMID: https://pubmed.ncbi.nlm.nih.gov/8018788/
5. Siegel A (2015) Fatal water intoxication and cardiac arrest in runners during marathons: prevention and treatment based on validated clinical paradigms. The American Journal of Medicine 128:1070-1075. PMID: https://pubmed.ncbi.nlm.nih.gov/25910792/
6. Levy T (2002) Curing the Incurable: Vitamin C, Infectious Diseases, and Toxins, Henderson, NV: MedFox Publishing
7. Levy T (2021) http://orthomolecular.org/resources/omns/v17n28.shtml
8. Levy T, Hunninghake R (2022) http://orthomolecular.org/resources/omns/v18n14.shtml
9. Ryu J, Sohn I, Do S (2009) Controlled hypotension for middle ear surgery: a comparison between remifentanil and magnesium sulphate. British Journal of Anaesthesia 103:490-495. PMID: https://pubmed.ncbi.nlm.nih.gov/19687032/
10. Jangra K, Malhotra S, Gupta A, Arora S (2016) Comparison of quality of the surgical field after controlled hypotension using esmolol and magnesium sulfate during endoscopic surgery. Journal of Anaesthesiology, Clinical Pharmacology 32:325-328. PMID: https://pubmed.ncbi.nlm.nih.gov/27625479/
11. Juibari H, Eftekharian H Arabion H (2016) Intravenous magnesium sulfate to deliberate hypotension and bleeding after bimaxillary orthognathic surgery; a randomized double-blind controlled trial. Journal of Dentistry 17:276-282. PMID: https://pubmed.ncbi.nlm.nih.gov/27840841/
12. Woods K, Fletcher S (1994) Long-term outcome after intravenous magnesium sulphate in suspected acute myocardial infarction: the second Leicester Intravenous Magnesium Intervention Trial (LIMIT-2). Lancet 343:816-819. PMID: https://pubmed.ncbi.nlm.nih.gov/7908076/
13. Shechter M, Hod H, Rabinowitz B et al. (2003) Long-term outcome of intravenous magnesium therapy in thrombolysis-ineligible acute myocardial infarction patients. Cardiology 99:205-210. PMID: https://pubmed.ncbi.nlm.nih.gov/12845247/
14. Levy T (2019) Magnesium: Reversing Disease, Henderson, NV: MedFox Publishing [For a free download of the eBook: https://mag.medfoxpub.com/]
15. Huang Y, Lee M, Wahlqvist M (2012) Prediction of all-cause mortality by B group vitamin status in the elderly. Clinical Nutrition 31:191-198. PMID: https://pubmed.ncbi.nlm.nih.gov/22071291/
16. Ricci C, Freisling H, Leitzmann M et al. (2020) Diet and sedentary behaviour in relation to cancer survival. A report from the national health and nutrition examination survey linked to the U.S. mortality registry. Clinical Nutrition 39:3489-3496. PMID: https://pubmed.ncbi.nlm.nih.gov/32229168/
17. Imdad A, Mayo-Wilson E, Haykal M et al. (2022) Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. The Cochrane Database of Systematic Reviews 3:CD008524. PMID: https://pubmed.ncbi.nlm.nih.gov/35294044/
18. Shea M, Barger K, Booth S et al. (2022) Vitamin K status, all-cause mortality, and cardiovascular disease in adults with chronic kidney disease: the Chronic Renal Insufficiency Cohort. The American Journal of Clinical Nutrition 115:941-948. PMID: https://pubmed.ncbi.nlm.nih.gov/34788785/
19. Wang J, Fan J, Yang Y et al. (2022) Vitamin D status and risk of all-cause and cause-specific mortality in osteoarthritis patients: results from NHANES III and NHANES 2001-2008. Nutrients 14:4629. PMID: https://pubmed.ncbi.nlm.nih.gov/36364891/
20. Xu K, Peng R, Zou Y et al. (2022) Vitamin C intake and multiple health outcomes: an umbrella review of systematic reviews and meta-analyses. International Journal of Food Sciences and Nutrition 73:588-599. PMID: https://pubmed.ncbi.nlm.nih.gov/35291895/
21. Hong Y, Zhou Z, Zhang N et al. (2022) Association between plasma vitamin B5 levels and all-cause mortality: a nested case-control study. Journal of Clinical Hypertension 24:945-954. PMID: https://pubmed.ncbi.nlm.nih.gov/35699663/
22. Liu Y, Geng T, Wan Z et al. (2022) Associations of serum folate and vitamin B12 levels with cardiovascular disease mortality among patients with type 2 diabetes. JAMA Network Open 5:e2146124. PMID: https://pubmed.ncbi.nlm.nih.gov/35099545/
23. Xu Q, Qian X, Sun F et al. (2023) Independent and joint associations of dietary antioxidant intake with risk of post-stroke depression and all-cause mortality. Journal of Affective Disorders 322:84-90. PMID: https://pubmed.ncbi.nlm.nih.gov/36372128/
24. Holubiec P, Leonczyk M, Staszewski F et al. (2021) Pathophysiology and clinical management of pellagra-a review. Folia Medica Cracoviensia 61:125-137. PMID: https://pubmed.ncbi.nlm.nih.gov/34882669/
25. Fricker R, Green E, Jenkins S, Griffin S (2018) The influence of nicotinamide on health and disease in the central nervous system. International Journal of Tryptophan Research 11:1-11. PMID: https://pubmed.ncbi.nlm.nih.gov/29844677/
26. Vignier N, Chatzifrangkeskou M, Rodriguez B et al. (2018) Rescue of biosynthesis of nicotinamide adenine dinucleotide protects the heart in cardiomyopathy caused by lamin A/C gene mutation. Human Molecular Genetics 27:3870-3880. PMID: https://pubmed.ncbi.nlm.nih.gov/30053027/
27. Bonora M, Patergnani S, Rimessi A et al. (2012) ATP synthesis and storage. Purinergic Signalling 8:343-357. PMID: https://pubmed.ncbi.nlm.nih.gov/22528680/
28. Lopez-Otin C, Blasco M, Partridge L et al. (2013) The hallmarks of aging. Cell 153:1194-1217. PMID: https://pubmed.ncbi.nlm.nih.gov/23746838/
29. Fang E, Lautrup S, Hou Y et al. (2017) NAD+ in aging: molecular mechanisms and translational implications. Trends in Molecular Medicine 23:899-916. PMID: https://pubmed.ncbi.nlm.nih.gov/28899755/
30. Imai S, Guarente L (2014) NAD+ and sirtuins in aging and disease. Trends in Cell Biology 24:464-471. PMID: https://pubmed.ncbi.nlm.nih.gov/24786309/
31. Yoshino J, Baur J, Imai S (2018) NAD+ intermediates: the biology and therapeutic potential of NMN and NR. Cell Metabolism 27:513-528. PMID: https://pubmed.ncbi.nlm.nih.gov/29249689/
32. Mouchiroud L, Houtkooper R, Moullan N et al. (2013) The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154:430-441. PMID: https://pubmed.ncbi.nlm.nih.gov/23870130/
33. Sohal R, Arnold L, Orr W (1990) Effect of age on superoxide dismutase, catalase, glutathione reductase, inorganic peroxides, TBA-reactive material, GSH/GSSG, NADPH/NADP+ and NADH/NAD+ in Drosophila melanogaster. Mechanisms of Ageing and Development 56:223-235. PMID: https://pubmed.ncbi.nlm.nih.gov/2128525/
34. Verdin E (2015) NAD+ in aging, metabolism, and neurodegeneration. Science 350:1208-1213. PMID: https://pubmed.ncbi.nlm.nih.gov/26785480/
35. Altschul R, Hoffer A, Stephen J (1955) Influence of nicotinic acid on serum cholesterol in man. Archives of Biochemistry and Biophysics 54:558-559. PMID: https://pubmed.ncbi.nlm.nih.gov/14350806/
36. Parsons Jr W, Achor R, Berge K et al. (1956) Changes in concentration of blood lipids following prolonged administration of large doses of nicotinic acid to persons with hypercholesterolemia: preliminary observations. Proceedings of the Staff Meetings. Mayo Clinic 31:377-390. PMID: https://pubmed.ncbi.nlm.nih.gov/13336128/
37. Parsons Jr W (2000) Introduction of niacin as the first successful treatment for cholesterol control, a reminiscence. Journal of Orthomolecular Medicine 15:121-126. http://orthomolecular.org/library/jom/2000/pdf/2000-v15n03-p121.pdf
38. Ganji S, Kamanna V, Kashyap M (2003) Niacin and cholesterol: role in cardiovascular disease (review). The Journal of Nutritional Biochemistry 14:298-305. PMID: https://pubmed.ncbi.nlm.nih.gov/12873710/
39. Huber R, Wong A (2020) Nicotinamide: an update and review of safety & differences from niacin. Skin Therapy Letter 25:7-11. PMID: https://pubmed.ncbi.nlm.nih.gov/33196157/
40. Parsons Jr W (2003) Cholesterol Control without Diet! The Niacin Solution. 2nd ed., Scottsdale, AZ: Lilac Press
41. Filler K, Lyon D, Bennett J et al. (2014) Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clinical 1:12-23. PMID: https://pubmed.ncbi.nlm.nih.gov/25147756/
42. Pirinen E, Auranen M, Khan N et al. (2020) Niacin cures systemic NAD+ deficiency and improves muscle performance in adult-onset mitochondrial myopathy. Cell Metabolism 31:1078-1090. PMID: https://pubmed.ncbi.nlm.nih.gov/32386566/
43. Schwemmlein J, Maack C, Bertero E (2022) Mitochondria as therapeutic targets in heart failure. Current Heart Failure Reports 19:27-37. PMID: https://pubmed.ncbi.nlm.nih.gov/35147851/
44. Starling R, Hammer D, Altschuld (1998) Human myocardial ATP content and in vivo contractile function. Molecular and Cellular Biochemistry 180:171-177. PMID: https://pubmed.ncbi.nlm.nih.gov/9546644/
45. Tannous C, Booz G, Altara R et al. (2021) Nicotinamide adenine dinucleotide: biosynthesis, consumption and therapeutic role in cardiac diseases. Acta Physiologica 231:e13551. PMID: https://pubmed.ncbi.nlm.nih.gov/32853469/
46. Kalsi K, Smolenski R, Pritchard R et al. (1999) Energetics and function of the failing human heart with dilated or hypertrophic cardiomyopathy. European Journal of Clinical Investigation 29:469-477. PMID: https://pubmed.ncbi.nlm.nih.gov/10354207/
47. Diguet N, Trammell S, Tannous C et al. (2018) Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 137:2256-2273. PMID: https://pubmed.ncbi.nlm.nih.gov/29217642/
48. Abdellatif M, Sedej S, Kroemer G (2021) NAD+ metabolism in cardiac health, aging, and disease. Circulation 144:1795-1817. PMID: https://pubmed.ncbi.nlm.nih.gov/34843394/
49. Trueblood N, Ramasamy R, Wang L, Schaefer S (2000) Niacin protects the isolated heart from ischemia-reperfusion injury. American Journal of Physiology. Heart and Circulatory Physiology 279:H764-H771. PMID: https://pubmed.ncbi.nlm.nih.gov/10924076/
50. Abdellatif M, Trummer-Herbst V, Koser F et al. (2021) Nicotinamide for the treatment of heart failure with preserved ejection fraction. Science Translational Medicine 13:eabd7064. PMID: https://pubmed.ncbi.nlm.nih.gov/33568522/
51. Bays H, Rader D (2009) Does nicotinic acid (niacin) lower blood pressure? International Journal of Clinical Practice 63:151-159. PMID: https://pubmed.ncbi.nlm.nih.gov/19054161/
52. Zhu X, Li J, Wang H et al. (2023) Nicotinamide restores tissue NAD+ and improves survival in rodent models of cardiac arrest. PLoS One 18:e0291598. PMID: https://pubmed.ncbi.nlm.nih.gov/37713442/
53. Airhart S, Shireman L, Risler L et al. (2017) An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS One 12:e0186459. PMID: https://pubmed.ncbi.nlm.nih.gov/29211728/
54. Hootkooper R, Canto C, Wanders R, Auwerx J (2010) The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocrine Reviews 31:194-223. PMID: https://pubmed.ncbi.nlm.nih.gov/20007326/
55. Canto C, Menzies K, Auwerx J (2015) NAD(+) metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metabolism 22:31-53. PMID: https://pubmed.ncbi.nlm.nih.gov/26118927/
56. Jacobson M, Jacobson E (2018) Vitamin B3 in health and disease: toward the second century of discovery. Methods in Molecular Biology 1813:3-8. PMID: https://pubmed.ncbi.nlm.nih.gov/30097857/
57. Rotllan N, Camacho M, Tondo M et al. (2018) Therapeutic potential of emerging NAD+-increasing strategies for cardiovascular diseases. Antioxidants 10:1939. PMID: https://pubmed.ncbi.nlm.nih.gov/34943043/
58. Mehmel M, Jovanovic N, Spitz U (2020) Nicotinamide riboside-the current state of research and therapeutic uses. Nutrients 12:1616. PMID: https://pubmed.ncbi.nlm.nih.gov/32486488/
59. Sharma C, Donu D, Cen Y (2022) Emerging role of nicotinamide riboside in health and diseases. Nutrients 14:3889. PMID: https://pubmed.ncbi.nlm.nih.gov/36235542/
60. Hong G, Zheng D, Zhang L et al. (2018) Administration of nicotinamide riboside prevents oxidative stress and organ injury in sepsis. Free Radical Biology & Medicine 123:125-137. PMID: https://pubmed.ncbi.nlm.nih.gov/29803807/
61. Poljsak B, Kovac V, Dahmane R et al. (2019) Cancer etiology: a metabolic disease originating from life’s major evolutionary transition? Oxidative Medicine and Cellular Longevity 2019:7831952. PMID: https://pubmed.ncbi.nlm.nih.gov/31687086/
62. Shah G, Shah R, Veillette H et al. (2005) Biochemical assessment of niacin deficiency among carcinoid cancer patients. The American Journal of Gastroenterology 100:2307-2314. PMID: https://pubmed.ncbi.nlm.nih.gov/16181385/
63. Kirkland J (2003) Niacin and carcinogenesis. Nutrition and Cancer 46:110-118. PMID: https://pubmed.ncbi.nlm.nih.gov/14690785/
64. Chen A, Martin A, Choy B et al. (2015) A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. The New England Journal of Medicine 373:1618-1626. PMID: https://pubmed.ncbi.nlm.nih.gov/26488693/
65. Benavente C, Jacobson M, Jacobson E (2009) NAD in skin: therapeutic approaches for niacin. Current Pharmaceutical Design 15:29-38. PMID: https://pubmed.ncbi.nlm.nih.gov/19149600/
66. Canner P, Berge K, Wenger N et al. (1986) Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. Journal of the American College of Cardiology 8:1245-1255. PMID: https://pubmed.ncbi.nlm.nih.gov/3782631/
67. Liu W, Cao S, Shi D et al. (2023) Association between dietary vitamin intake and mortality in US adults with diabetes: a prospective cohort study. Diabetes/Metabolism Research and Reviews Sep 26. Online ahead of print. PMID: https://pubmed.ncbi.nlm.nih.gov/37750562/
68. Langsjoen P, Folkers K, Lyson et al. (1990) Pronounced increase in survival of patients with cardiomyopathy when treated with coenzyme Q10 and conventional therapy. International Journal of Tissue Reactions 12:163-168. PMID: https://pubmed.ncbi.nlm.nih.gov/2276894/
69. Langsjoen P, Langsjoen A (2008) Supplemental ubiquinol in patients with advanced congestive heart failure. Biofactors 32:119-128. PMID: https://pubmed.ncbi.nlm.nih.gov/19096107/
70. Oleck S, Ventura H (2016) Coenzyme Q10 and utility in heart failure: just another supplement? Current Heart Failure Reports 13:190-195. PMID: https://pubmed.ncbi.nlm.nih.gov/27333901/
71. Lei L, Liu Y (2017) Efficacy of coenzyme Q10 in patients with cardiac failure: a meta-analysis of clinical trials. BMC Cardiovascular Disorders 17:196. PMID: https://pubmed.ncbi.nlm.nih.gov/28738783/
72. Langsjoen P, Langsjoen A, Folkers K (1990) A six-year clinical study of therapy of cardiomyopathy with coenzyme Q10. International Journal of Tissue Reactions 12:169-171. PMID: https://pubmed.ncbi.nlm.nih.gov/2276895/
73. Mortensen S, Rosenfeldt F, Kumar A et al. (2014) The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC. Heart Failure 2:641-649. PMID: https://pubmed.ncbi.nlm.nih.gov/25282031/
74. Fotino A, Thompson-Paul A, Bazzano L (2013) Effect of coenzyme Q10 supplementation on heart failure: a meta-analysis. The American Journal of Clinical Nutrition 97:268-275. PMID: https://pubmed.ncbi.nlm.nih.gov/23221577/
75. Langsjoen P, Langsjoen A, Willis R, Folkers K (1997) Treatment of hypertrophic cardiomyopathy with coenzyme Q10. Molecular Aspects of Medicine 18 Suppl:S145-S151. PMID: https://pubmed.ncbi.nlm.nih.gov/9266516/
76. Sobirin M. Herry Y, Sofia S et al. (2019) Effects of coenzyme Q10 supplementation on diastolic function in patients with heart failure with preserved ejection fraction. Drug Discoveries & Therapeutics 13:38-46. PMID: https://pubmed.ncbi.nlm.nih.gov/30880321/
77. Yan H, Zou T, Tuo Q et al. (2021) Ferroptosis: mechanisms and links with diseases. Signal Transduction and Targeted Therapy 6:49. PMID: https://pubmed.ncbi.nlm.nih.gov/33536413/
78. Walker M, Tian R (2018) Raising NAD in heart failure: time to translate? Circulation 137:2274-2277. PMID: https://pubmed.ncbi.nlm.nih.gov/29784680/
79. Griffin S, Pickard M, Orme R et al. (2013) Nicotinamide promotes neuronal differentiation of mouse embryonic stem cells in vitro. Neuroreport 24:1041-1046. PMID: https://pubmed.ncbi.nlm.nih.gov/24257250/
80. Griffin S, Pickard M, Orme R et al. (2017) Nicotinamide alone accelerates the conversion of mouse embryonic stem cells into mature neuronal populations. PLoS One 12:e0183358. PMID: https://pubmed.ncbi.nlm.nih.gov/28817722/
81. Gasperi V, Sibilano M, Savini I, Catani M (2019) Niacin in the central nervous system: an update of biological aspects and clinical applications. International Journal of Molecular Sciences 20:974. PMID: https://pubmed.ncbi.nlm.nih.gov/30813414/
82. Spector R (1987) Niacinamide transport through the blood-brain barrier. Neurochemical Research 12:27-31. PMID: https://pubmed.ncbi.nlm.nih.gov/2952896/
83. Ito T, Sato T, Takanashi Y et al. (2021) A single oral supplementation of nicotinamide within the daily tolerable upper level increases blood NAD+ levels in healthy subjects. Translational Medicine of Aging 5:43-51. https://www.sciencedirect.com/science/article/pii/S2468501121000055
84. Cui X, Chopp M, Zacharek A et al. (2010) Niacin treatment of stroke increases synaptic plasticity and axon growth in rats. Stroke 20671245 https://pubmed.ncbi.nlm.nih.gov/20671245/
85. Shehadah A, Chen J, Zacharek A et al. (2010) Niaspan treatment induces neuroprotection after stroke. Neurobiology of Disease 40:277-283. PMID: https://pubmed.ncbi.nlm.nih.gov/20554037/
86. Maiese K, Chong Z (2003) Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends in Pharmacological Sciences 24:228-232. PMID: https://pubmed.ncbi.nlm.nih.gov/12767721/
87. Mokudai T, Ayoub I, Sakakibara Y et al. (2000) Delayed treatment with nicotinamide (vitamin B3) improves neurological outcome and reduces infarct volume after transient focal cerebral ischemia in Wistar rats. Stroke 31:1679-1685. PMID: https://pubmed.ncbi.nlm.nih.gov/10884473/
88. Suchard M, Savulescu D (2022) Nicotinamide pathways as the root cause of sepsis-an evolutionary perspective on macrophage energetic shifts. The FEBS Journal 289:955-964. PMID: https://pubmed.ncbi.nlm.nih.gov/33686748/
89. Kujundzic R (2022) COVID-19: are we facing secondary pellagra which cannot simply be cured by vitamin B3? International Journal of Molecular Sciences 23:4309. PMID: https://pubmed.ncbi.nlm.nih.gov/35457123/
90. Altay O, Arif M, Li X et al. (2021) Combined metabolic activators accelerates recovery in mild-to-moderate COVID-19. Advanced Sciences 8:e2101222. PMID: https://pubmed.ncbi.nlm.nih.gov/34180141/
91. Badawy A (2020) Immunotherapy of COVID-19 with poly (ADP-ribose) polymerase inhibitors: starting with nicotinamide. Bioscience Reports 40:BSR20202856. PMID: https://pubmed.ncbi.nlm.nih.gov/33063092/
92. Raines N, Ganatra S, Nissaisorakarn P et al. (2020) Niacinamide may be associated with improved outcomes in COVID-19-related acute kidney injury: an observational study. Kidney360 2:33-41. PMID: https://pubmed.ncbi.nlm.nih.gov/35368823/
93. Kumakura S, Sato E, Sekimoto A et al. (2021) Nicotinamide attenuates the progression of renal failure in a mouse model of adenine-induced chronic kidney disease. Toxins 13:50. PMID: https://pubmed.ncbi.nlm.nih.gov/33440677/
94. Brown O, Heitkamp M, Song C (1981) Niacin reduces paraquat toxicity in rats. Science 212:1510-1512. PMID: https://pubmed.ncbi.nlm.nih.gov/7233236/
95. Hoffer A (1975) Nutrition and schizophrenia. Canadian Family Physician 21:78-82. PMID: https://pubmed.ncbi.nlm.nih.gov/20469184/
96. Morris M, Schneider J, Tangney C (2006) Thoughts on B-vitamins and dementia. Journal of Alzheimer’s Disease 9:429-433. PMID: https://pubmed.ncbi.nlm.nih.gov/16917152/
97. Rivadeneira A, Moyer P, Salciccioli J (2019) Pellagra in the USA: unusual manifestations of a rare entity. BMJ Case Reports 12:e230972. PMID: https://pubmed.ncbi.nlm.nih.gov/31570356/
98. Morris M, Evans D, Bienias J et al. (2004) Dietary niacin and the risk of incident Alzheimer’s disease and of cognitive decline. Journal of Neurology, Neurosurgery, and Psychiatry 75:1093-1099. PMID: https://pubmed.ncbi.nlm.nih.gov/15258207/
99. Wakade C, Chong R, Bradley E et al. (2014) Upregulation of GPR109A in Parkinson’s disease. PLoS One 9:e109818. PMID: https://pubmed.ncbi.nlm.nih.gov/25329911/
100. Hellenbrand W, Boeing H, Robra B et al. (1996) Diet and Parkinson’s disease. II. A possible role for the past intake of specific nutrients. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology 47:644-650. PMID: https://pubmed.ncbi.nlm.nih.gov/8797457/
101. Fall P, Fredrikson M, Axelson O, Granerus A (1999) Nutritional and occupational factors influencing the risk of Parkinson’s disease: a case-control study in southeastern Sweden. Movement Disorders 14:28-37. PMID: https://pubmed.ncbi.nlm.nih.gov/9918341/
102. Hoffer L (2008) Vitamin therapy in schizophrenia. The Israel Journal of Psychiatry and Related Sciences 45:3-10. PMID: https://pubmed.ncbi.nlm.nih.gov/18587164/
103. Pasternak O, Kubicki M, Shenton M (2016) In vivo imaging of neuroinflammation in schizophrenia. Schizophrenia Research 173:200-212. PMID: https://pubmed.ncbi.nlm.nih.gov/26048294/
104. Ansari Z, Pawar S, Seetharaman R (2022) Neuroinflammation and oxidative stress in schizophrenia: are these opportunities for repurposing? Postgraduate Medicine 134:187-199. PMID: https://pubmed.ncbi.nlm.nih.gov/34766870/
105. Meyer U (2013) Developmental neuroinflammation and schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry 42:20-34. PMID: https://pubmed.ncbi.nlm.nih.gov/22122877/
106. Karlsson H, Dalman C (2020) Epidemiological studies of prenatal and childhood infection and schizophrenia. Current Topics in Behavioral Neurosciences 44:35-47. PMID: https://pubmed.ncbi.nlm.nih.gov/30852763/
107. Rund B (2018) The research evidence for schizophrenia as a neurodevelopmental disorder. Scandinavian Journal of Psychology 59:49-58. PMID: https://pubmed.ncbi.nlm.nih.gov/29356007/
108. Falkai P, Schmitt A (2022) Failed regeneration and inflammation in schizophrenia: two sides of the same coin? Journal of Neural Transmission 129:611-615. PMID: https://pubmed.ncbi.nlm.nih.gov/35451657/
109. Shah D, Pandey S, Rathi R (1972) Psychiatric manifestations in pellagra. The Journal of the Association of Physicians of India 20:575-578. PMID: https://pubmed.ncbi.nlm.nih.gov/4680826/
110. Periyasamy S, John S, Padmavati R et al. (2019) Association of schizophrenia risk with disordered niacin metabolism in an Indian genome-wide association study. JAMA Psychiatry 76:1026-1034. PMID: https://pubmed.ncbi.nlm.nih.gov/31268507/
111. Prakash R, Gandotra S, Singh L et al. (2008) Rapid resolution of delusional parasitosis in pellagra with niacin augmentation therapy. General Hospital Psychiatry 30:581-584. PMID: https://pubmed.ncbi.nlm.nih.gov/19061687/
112. Mahler M, Cummings J, Benson D (1987) Treatable dementias. The Western Journal of Medicine 146:705-712. PMID: https://pubmed.ncbi.nlm.nih.gov/3617715/
113. Xu X, Jiang G (2015) Niacin-respondent subset of schizophrenia-a therapeutic review. European Review for Medical and Pharmacological Sciences 19:988-997. PMID: https://pubmed.ncbi.nlm.nih.gov/25855923/
114. Torrey E, Yolken R (2003) Toxoplasma gondii and schizophrenia. Emerging Infectious Diseases 9:1375-1380. PMID: https://pubmed.ncbi.nlm.nih.gov/14725265/
115. Rantala M, Luoto S, Borraz-Leon J, Krams I (2022) Schizophrenia: the new etiological synthesis. Neuroscience and Biobehavioral Reviews 142:104894. PMID: https://pubmed.ncbi.nlm.nih.gov/36181926/
116. Miklossy J, Khalili K, Gern L et al. (2004) Borrelia burgdorferi persists in the brain in chronic Lyme neuroborreliosis and may be associated with Alzheimer’s disease. Journal of Alzheimer’s Disease 6:673-681. PMID: https://pubmed.ncbi.nlm.nih.gov/15665404/
117. Sasmita A (2019) Modification of the gut microbiome to combat neurodegeneration. Reviews in the Neurosciences 30:795-805. PMID: https://pubmed.ncbi.nlm.nih.gov/31095511/
118. Wozniak M, Mee A, Itzhaki R (2009) Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. The Journal of Pathology 217:131-138. PMID: https://pubmed.ncbi.nlm.nih.gov/18973185/
119. Alonso R, Pisa D, Marina A et al. (2014) Fungal infection in patients with Alzheimer’s disease. Journal of Alzheimer’s Disease 41:301-311. PMID: https://pubmed.ncbi.nlm.nih.gov/24614898/
120. Dominy S, Lynch C, Ermini F et al. (2019) Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Science Advances 5:eaau3333. PMID: https://pubmed.ncbi.nlm.nih.gov/30746447/
121. Balin B, Gerard H, Arking E et al. (1998) Identification and localization of Chlamydia pneumoniae in the Alzheimer’s brain. Medical Microbiology and Immunology 187:23-42. PMID: https://pubmed.ncbi.nlm.nih.gov/9749980/
122. Jiang C, Li G, Huang P et al. (2017) The gut microbiota and Alzheimer’s disease. Journal of Alzheimer’s Disease 58:1-15. PMID: https://pubmed.ncbi.nlm.nih.gov/28372330/
123. Fang X (2016) Potential role of gut microbiota and tissue barriers in Parkinson’s disease and amyotrophic lateral sclerosis. The International Journal of Neuroscience 126:771-776. PMID: https://pubmed.ncbi.nlm.nih.gov/26381230/
124. Ning J, Huang S, Chen S et al. (2022) Investigating casual associations among gut microbiota, metabolites, and neurodegenerative diseases: a Mendelian randomization study. Journal of Alzheimer’s Disease 87:211-222. PMID: https://pubmed.ncbi.nlm.nih.gov/35275534/
125. Zhuang Z, Yang R, Wang W et al. (2020) Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. Journal of Neuroinflammation 17:288. PMID: https://pubmed.ncbi.nlm.nih.gov/33008395/
126. Picchioni M, Murray R (2007) Schizophrenia. BMJ 335:91-95. PMID: https://pubmed.ncbi.nlm.nih.gov/17626963/
127. Madsen A, Keidling N, Karle A et al. (1998) Neuroleptics in progressive structural brain abnormalities in psychiatric illness. Lancet 352:784-785. PMID: https://pubmed.ncbi.nlm.nih.gov/9737286/
128. Cahn W, Pol H, Lems E et al. (2002) Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Archives of General Psychiatry 59:1002-1010. PMID: https://pubmed.ncbi.nlm.nih.gov/12418933/
129. Emsley R, Asmal L, du Plessis S et al. (2017) Brain volume changes over the first year of treatment in schizophrenia: relationships to antipsychotic treatment. Psychological Medicine 47:2187-2196. PMID: https://pubmed.ncbi.nlm.nih.gov/28347393/
130. Hirayasu Y (2007) Brain imaging in schizophrenia. Neuropathology 27:601-603. PMID: https://pubmed.ncbi.nlm.nih.gov/18021383/
131. Muller N (2018) Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophrenia Bulletin 44:973-982. PMID: https://pubmed.ncbi.nlm.nih.gov/29648618/
132. Marques T, Ashok A, Pillinger T et al. (2019) Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychological Medicine 49:2186-2196. PMID: https://pubmed.ncbi.nlm.nih.gov/30355368/
133. Leng F, Edison P (2021) Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nature Reviews Neurology 17:157-172. PMID: https://pubmed.ncbi.nlm.nih.gov/33318676/
134. Na K, Jung H, Kim Y (2014) The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Progress in Neuro-Psychopharmacology & Biological Psychiatry 48:277-286. PMID: https://pubmed.ncbi.nlm.nih.gov/23123365/
135. Buckley P (2019) Neuroinflammation and schizophrenia. Current Psychiatry Reports 21:72. PMID: https://pubmed.ncbi.nlm.nih.gov/31267432/
136. Murphy C, Walker A, Weickert C (2021) Neuroinflammation in schizophrenia: the role of nuclear factor kappa B. Translational Psychiatry 11:528. PMID: https://pubmed.ncbi.nlm.nih.gov/34650030
137. Bechter (2013) [Schizophrenia-a mild encephalitis?] Article in German. Fortschritte der Neurologie-Psychiatrie 81:250-259. PMID: https://pubmed.ncbi.nlm.nih.gov/23629631/
138. Osmond H, Hoffer A (1959) A small research in schizophrenia. Canadian Medical Association Journal 80:91-94. PMID: https://pubmed.ncbi.nlm.nih.gov/13618799/
139. Lahteenvuo M, Tiihonen J (2021) Antipsychotic polypharmacy for the management of schizophrenia: evidence and recommendations. Drugs 81:1273-1284. PMID: https://pubmed.ncbi.nlm.nih.gov/34196945/
140. Weller M (1987) The spectrum of schizophrenia. Postgraduate Medical Journal 63:1021-1024. PMID: https://pubmed.ncbi.nlm.nih.gov/3330234/
141. Hoffer A, Osmond H, Callbeck M, Kahan I (1957) Treatment of schizophrenia with nicotinic acid and nicotinamide. Journal of Clinical and Experimental Psychopathology 18:131-158. PMID: https://pubmed.ncbi.nlm.nih.gov/13439009/
142. Sandyk R, Kanofsky J (1993) Vitamin C in the treatment of schizophrenia. The International Journal of Neuroscience 68:67-71. PMID: https://pubmed.ncbi.nlm.nih.gov/8063516/
143. Hoffer A, Prousky J (2008) The proper treatment of schizophrenia requires optimal daily doses of vitamin B3. Journal of Orthomolecular Medicine 23:191-195. http://orthomolecular.org/library/jom/2008/pdf/2008-v23n04-p191.pdf
144. Hoffer A, Prousky J (2008) Successful treatment of schizophrenia requires optimal daily doses of vitamin B3. Alternative Medicine Review 13:287-291. PMID: https://pubmed.ncbi.nlm.nih.gov/19238764/
145. Hoffer A (1994) Chronic schizophrenic patients treated ten years or more. Journal of Orthomolecular Medicine 9:7-37.
146. Hoffer A, Osmond H (1964) Treatment of schizophrenia with nicotinic acid. A ten year follow-up. Acta Psychiatrica Scandinavica 40:171-189. PMID: https://pubmed.ncbi.nlm.nih.gov/14235254/
147. Hoffer A (1963) Nicotinic acid: an adjunct in the treatment of schizophrenia. The American Journal of Psychiatry 120:171-173. PMID: https://pubmed.ncbi.nlm.nih.gov/13963912/
148. Osmond H, Hoffer A (1962) Massive niacin treatment in schizophrenia: review of a nine-year study. Lancet 1:316-319. PMID: https://pubmed.ncbi.nlm.nih.gov/14482545/
149. Hoffer A, Saul A, Foster H (2023) Niacin: The Real Story. Nashville, TN: Basic Health Publications, Inc.
150. Hoffer A (2020) Light on Schizophrenia: Revealing causes and solutions from an orthomolecular perspective. Victoria, BC: Tellwell Publishing
151. Hoffer A (1971) Megavitamin B-3 therapy for schizophrenia. Canadian Psychiatric Association Journal 16:499-504. PMID: https://pubmed.ncbi.nlm.nih.gov/4947171/
152. Hoffer A (1973) A neurological form of schizophrenia. Canadian Medical Association Journal 108:186. PMID: https://pubmed.ncbi.nlm.nih.gov/4684627/
153. Premkumar V, Yuvaraj S, Vijayasarathy K et al. (2007) Effect of coenzyme Q10, riboflavin and niacin on serum CEA and CA 15-3 levels in breast cancer patients undergoing tamoxifen therapy. Biological & Pharmaceutical Bulletin 30:367-370. PMID: https://pubmed.ncbi.nlm.nih.gov/17268082/
154. Yuvaraj S, Premkumar V, Vijayasarathy K et al. (2008) Augmented antioxidant status in tamoxifen treated postmenopausal women with breast cancer on co-administration with coenzyme Q10, niacin and riboflavin. Cancer Chemotherapy and Pharmacology 61:933-941. PMID: https://pubmed.ncbi.nlm.nih.gov/17668211/
155. Premkumar V, Yuvaraj S, Sathish S et al. (2008) Anti-angiogenic potential of Coenzyme Q10, riboflavin and niacin in breast cancer patients undergoing tamoxifen therapy. Vascular Pharmacology 48:191-201. PMID: https://pubmed.ncbi.nlm.nih.gov/18407793/
Nutritional Medicine is Orthomolecular Medicine
Orthomolecular medicine uses safe, effective nutritional therapy to fight illness. For more information: http://www.orthomolecular.org
Find a Doctor
To locate an orthomolecular physician near you: http://orthomolecular.org/resources/omns/v06n09.shtml
The peer-reviewed Orthomolecular Medicine News Service is a non-profit and non-commercial informational resource.
Editorial Review Board:
Albert G. B. Amoa, MB.Ch.B, Ph.D. (Ghana)
Seth Ayettey, M.B., Ch.B., Ph.D. (Ghana)
Ilyès Baghli, M.D. (Algeria)
Barry Breger, M.D. (Canada)
Ian Brighthope, MBBS, FACNEM (Australia)
Gilbert Henri Crussol, D.M.D. (Spain)
Carolyn Dean, M.D., N.D. (USA)
Ian Dettman, Ph.D. (Australia)
Susan R. Downs, M.D., M.P.H. (USA)
Ron Ehrlich, B.D.S. (Australia)
Hugo Galindo, M.D. (Colombia)
Gary S. Goldman, Ph.D. (USA)
William B. Grant, Ph.D. (USA)
Claus Hancke, MD, FACAM (Denmark)
Patrick Holford, BSc (United Kingdom)
Ron Hunninghake, M.D. (USA)
Bo H. Jonsson, M.D., Ph.D. (Sweden)
Dwight Kalita, Ph.D. (USA)
Felix I. D. Konotey-Ahulu, M.D., FRCP (Ghana)
Peter H. Lauda, M.D. (Austria)
Fabrice Leu, N.D., (Switzerland)
Alan Lien, Ph.D. (Taiwan)
Homer Lim, M.D. (Philippines)
Stuart Lindsey, Pharm.D. (USA)
Pedro Gonzalez Lombana, M.D., Ph.D. (Colombia)
Victor A. Marcial-Vega, M.D. (Puerto Rico)
Juan Manuel Martinez, M.D. (Colombia)
Mignonne Mary, M.D. (USA)
Joseph Mercola, D.O. (USA)
Jorge R. Miranda-Massari, Pharm.D. (Puerto Rico)
Karin Munsterhjelm-Ahumada, M.D. (Finland)
Sarah Myhill, MB, BS (United Kingdom)
Tahar Naili, M.D. (Algeria)
Zhiyong Peng, M.D. (China)
Isabella Akyinbah Quakyi, Ph.D. (Ghana)
Selvam Rengasamy, MBBS, FRCOG (Malaysia)
Jeffrey A. Ruterbusch, D.O. (USA)
Gert E. Schuitemaker, Ph.D. (Netherlands)
Thomas N. Seyfried, Ph.D. (USA)
Han Ping Shi, M.D., Ph.D. (China)
T.E. Gabriel Stewart, M.B.B.CH. (Ireland)
Jagan Nathan Vamanan, M.D. (India)
Andrew W. Saul, Ph.D. (USA), Editor-In-Chief
Associate Editor: Robert G. Smith, Ph.D. (USA)
Editor, Japanese Edition: Atsuo Yanagisawa, M.D., Ph.D. (Japan)
Editor, Chinese Edition: Richard Cheng, M.D., Ph.D. (USA)
Editor, Norwegian Edition: Dag Viljen Poleszynski, Ph.D. (Norway)
Editor, Arabic Edition: Moustafa Kamel, R.Ph, P.G.C.M (Egypt)
Editor, Korean Edition: Hyoungjoo Shin, M.D. (South Korea)
Editor, Spanish Edition: Sonia Rita Rial, PhD (Argentina)
Editor, German Edition: Bernhard Welker, M.D. (Germany)
Associate Editor, German Edition: Gerhard Dachtler, M.Eng. (Germany)
Assistant Editor: Michael Passwater (USA)
Contributing Editor: Thomas E. Levy, M.D., J.D. (USA)
Contributing Editor: Damien Downing, M.B.B.S., M.R.S.B. (United Kingdom)
Contributing Editor: W. Todd Penberthy, Ph.D. (USA)
Contributing Editor: Ken Walker, M.D. (Canada)
Contributing Editor: Michael J. Gonzalez, N.M.D., Ph.D. (Puerto Rico)
Technology Editor: Michael S. Stewart, B.Sc.C.S. (USA)
Associate Technology Editor: Robert C. Kennedy, M.S. (USA)
Legal Consultant: Jason M. Saul, JD (USA)
Comments and media contact: drsaul@doctoryourself.com OMNS welcomes but is unable to respond to individual reader emails. Reader comments become the property of OMNS and may or may not be used for publication.
Riordan Clinic | Orthomolecular.org
3100 N Hillside Ave
Wichita, Kansas 67219
United States
Can These Nutrients Help Prevent Muscle Wasting?
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2022/11/25/how-to-prevent-sarcopenia.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola Fact Checked November 25, 2022<
STORY AT-A-GLANCE
- Age-related muscle loss, also called sarcopenia, begins at age 30 and affects at least half of the people over age 80; omega-3 fatty acids, the amino acid leucine, and the probiotic Lactobacillus paracasei PS23 help counteract sarcopenia
- Whey protein is high in leucine and has long been known as an excellent source of protein that is easily digested and absorbed. Leucine may regulate muscle protein turnover and is an effective way to optimize muscle growth when combined with resistance training
- Nicotinamide adenine dinucleotide (NAD+) is a substrate for important enzymes and impacts age-related amyloid protein aggregates in the muscle that affects muscle aging. Boost your levels through exercise, sauna bathing, fasting, and minimizing EMF exposure
- A Harvard study showed consuming protein is not enough to protect muscle mass, participants must also include strength training, which also improves basal blood flow in the lower extremities. This helps protect against functional impairment and metabolic syndrome
As you age, your body naturally tends to lose muscle. This condition is known as sarcopenia or muscle wasting. A 2022 study published in the journal Nutrients1 found increasing certain nutrients could lower your risk of sarcopenia as you age.
If you are not proactive, you can expect to lose approximately 15% of your muscle mass between your 30s and your 80s.2 An estimated3 10% to 25% of older adults under age 70 and half of those over age 80 have sarcopenia.
Even when you’re younger, if you are forced to stay in bed it can have a dramatic impact on your muscle mass. In one 2015 review,4 researchers found you could lose 5.2% of your muscle mass in the first two weeks of bed rest and by Day 23, you could have lost up to 10% of your quadriceps muscle mass. Strong muscles are required for mobility, balance and the ability to live independently.
Sarcopenia5 can increase the risk of falls and fractures, which ultimately can lead to hospitalization and surgery. It is important to know that sarcopenia is not related to your body mass. In other words, individuals who are obese can also lose muscle mass, which increases their risk for complications.
One meta-analysis6 of 35 studies and 58,404 people demonstrated the global prevalence of sarcopenia is 10% in men and women. Scientists understand the importance of muscle wasting as it relates to longevity and health. This led the Centers for Disease Control and Prevention7 to recognize it as an independently reportable medical condition.
When you have a reserve of muscle mass, it minimizes the challenges that result from muscle wasting8 if you become sick or hospitalized. Because muscle is lost far more easily and quickly than it’s built, it’s crucial to find ways to continuously promote and maintain muscle mass.
Nutritional Treatment Helps Counteract Sarcopenia
Researchers in the featured study9 knew that a variety of nutrients have shown effectiveness in supporting muscle. The randomized clinical trial was developed to analyze how effective two months of food high in omega-3 fats, leucine and probiotic Lactobacillus paracasei PS23 (LPPS23) would be on appendicular lean mass, inflammatory status, amino acid profile and muscle performance in people who were diagnosed with sarcopenia.
The researchers enrolled 60 participants who were within 4.8 years of 79.7 years and split them into an intervention or placebo group. The researchers prepared a customized diet schedule for both groups that provided 1.5 grams of protein per kilogram of body weight each day.10 Each group also received a dietary plan that consisted of approximately 30% lipids and 55% carbohydrates.
The researchers measured weight loss as compared to the individual’s weight history in the six months before their baseline visit. The subjects were questioned about how well they had followed their diet plan and physical activity recommendations. They also filled out a 24-hour dietary recall once a month.
The group that received the intervention took a supplement containing 500 mg of omega-3 fatty acid, 2.5 g of leucine and an LPPS23 probiotic. The control group received an isocaloric placebo. At the end of the study, the researchers measured body composition, physical performance, mood, blood pressure, muscle strength and functional status.
They concluded from the measurements that the intervention appeared to be a “valid strategy to counteract the progression of sarcopenia and sarcopenic-defining parameters in older adults.”11
Whey Protein Another Tool to Prevent Sarcopenia
As the featured study demonstrated, your diet plays a significant role in muscle development since your muscles need enough protein to stay viable. A 2011 paper12 published in the American Journal of Nutrition noted that the differences in digestion and absorption of dietary protein can modulate muscle growth.
So, while you need protein to build and maintain muscle, some proteins are more easily digested and absorbed than others. When you eat the right kind of protein it can make a difference in the potential risk for sarcopenia. The researchers in the featured study13 included the amino acid leucine in the intervention, which is also found in high concentrations in whey protein.14
Whey is a byproduct of cheese production and has long been acknowledged as an excellent source of protein. In the 2011 study, whey protein was compared to casein and casein hydrolysate, and was found to stimulate muscle protein growth the best, likely because of the leucine content.
One of the reasons that leucine is so important to prevent sarcopenia is that it helps regulate the turnover of protein in your muscle. A 1975 paper15 in the Journal of Clinical Investigation said leucine may also “play a pivotal role in the protein-sparing effect of amino acids.”
A more recent 2017 study16 explained the most effective way to optimize muscle building is to use a combination of resistance training followed by a protein meal, with leucine-rich whey being one of the most efficient proteins that can be used. However, a Harvard study demonstrated that simply taking leucine will likely be ineffective.17
Two groups of men over age 65 consumed either 0.8 grams of protein per kilo per day or 1.3 g of protein per kilo per day. The researchers found the high protein group did not experience an increase in lean muscle mass, or improve physical function or muscle strength, greater than the low protein group, most likely because they were not exercising.
Whey protein also contains glutathione, another important component in promoting and protecting muscle mass. It is thought to play an important role in muscle wasting, specifically in helping to modulate higher levels of oxidative stress18 often found in patients with sarcopenia. As noted in a 2012 review:19
“It has been suggested that oral antioxidant supplementation may contribute at reducing indices of oxidative stress both in animal and human models by reinforcing the natural endogenous defenses …
Antioxidants are substances able to inhibit the rate of oxidation. Mainly, antioxidant enzymes (e.g., catalase, superoxide dismutase (SOD), glutathione peroxidase, glutathione reductase) work to maintain a state of balance preventing the transformation of ROS and to convert them into more stable molecules (like water and molecular oxygen).”
NAD+ Combats Age-Related Muscle Deterioration
Studies have also proposed that mitochondrial dysfunction in the motor neurons may drive the development of sarcopenia. A 2021 paper20 published in Cell Reports compared the similarities between muscle aging and degenerative muscle diseases. The data revealed that protein aggregates deposit in skeletal muscle, which is a feature of muscle aging.
The researchers identified an amyloid-like protein that impairs mitochondrial function. While researchers have known that aggregated proteins could contribute to brain aging, this was the first time that data had shown it could contribute to muscle aging and directly damage the mitochondria.
The researchers first used a substance in worms and found it could reduce age-related amyloid protein aggregates.21 They found the same results in human muscle tissue from older subjects. They then went on to test nicotinamide adenine dinucleotide (NAD+) boosting nicotinamide riboside in aged mice and found it reduced the number and size of the amyloid aggregates within the skeletal muscle tissue.
NAD+ is a substrate for several important enzymes and is essential in metabolic processes, such as creating ATP in the mitochondria. A 2020 paper22 published in Endocrinology and Metabolism demonstrated that when the NAD+ salvage pathways in muscle are impaired, mitochondrial dysfunction and decreased muscle mass ensue.
If your NAD+ level is low, some simple lifestyle strategies can help. For example, exercise, fasting, minimizing electromagnetic field (EMF) exposure and sauna bathing can help improve your NAD+ levels. Exercise, heat exposure and fasting address low NAD+ because they are catabolic stressors that activate AMP protein kinase (AMPK).
This in turn activates an enzyme called NAMPT, which governs the NAD+ salvage pathway. Oxidative stress and inflammation can also deplete NAD+. Exercise, sauna and fasting can help reduce oxidative stress and inflammation, and as a result, less NAD+ is depleted.
The best way to increase NAD+ levels is to optimize your circadian rhythm by going to bed around 9 and getting up around 5 AM. The further you veer from these hours the more you challenge your circadian rhythm. It is also important to avoid blue light from screens and home lights after sunset and before sunrise.
Then you want to make sure you are doing regular strength training exercises. The best time to do them would be in the AM while you are fasting. You can also take niacinamide 50 mg powder three times a day. More is not better and will be counterproductive by inhibiting your longevity proteins (sirtuins). You can see my interview with Nichola Conlon for more details.
Strength Training Helps Preserve Muscle Mass and Heart Health
As the Harvard study23 demonstrated, consuming protein is not enough to protect your muscle mass. One study24 from the University of Michigan School of Public Health found those with lower muscle strength did not live as long as their peers with stronger muscles. After adjusting for confounding factors, the data continue to show those with low muscle strength had a 50% greater risk of dying early.
The data was pulled from a study of 8,326 men and women aged 65 and older. A loss of muscle in older adults may also be a primary driver of insulin resistance25 and declining strength may impact a reduction in daily physical activity, which also contributes to metabolic dysfunction.26
Researchers from the National Institute of Health and Nutrition in Tokyo, Japan,27 tested the hypothesis that a reduction in leg blood flow would be absent or minimal in people who regularly perform strength training exercises. They engaged a group of 104 men ages of 20 to 34 and 35 to 65 to compare whole-leg blood flow and vascular conductance between the groups.
The data showed no notable differences in the two age groups in those who used resistance training. However, there was a significant difference in the sedentary middle-aged group leading the team to conclude that a reduction in basal whole leg blood flow may be absent in men who routinely engage in resistance training.
The researchers suggested that resistance training could favorably influence leg perfusion. Lower levels of basal leg blood flow are associated with developing metabolic syndrome and functional impairment.28
I have been exercising for over 50 years. In the first 43 years, I exclusively used aerobic exercise. I didn’t realize that while it lowered the risk of heart disease, it is highly catabolic and eventually lowers the ability to build muscle. At the height of my running career, my upper arm circumference was 10.5 in.
However, contrast that with my arm circumference in December 2020 when it measured 15 inches. I stopped long-distance running and started resistance training. The results didn’t happen overnight, and I was well over 50 when I first began using resistance training.
The key to my success has been allowing time for significant recovery so the connective tissue and muscle can rebuild. I work with a trainer, but if you cannot afford a trainer, there are many great videos.
When you use resistance training and add the nutritional elements your body needs to grow muscles, you’ll reap the benefits. Remember to avoid doing the same exercise every day to allow the body to recover and repair so you get the benefits and avoid injuries.
- 1, 13 Nutrients, 2022;14(21)
- 2 Journals of Gerontology, 1995;50(5-8)
- 3 Journals of Gerontology, 2003; 58(10)
- 4 Extreme Physiology & Medicine 2015;4:16
- 5 Aging in Motion, What Is Sarcopenia?
- 6 Journal of Diabetes and Metabolic Disorders, 2017; 16
- 7 Federal Practitioner, 2017;34(7)
- 8 Current Opinions in Clinical Nutrition and Metabolic Care 2012; 15(1)
- 9 Nutrients, 2022;14(21) Abstract
- 10 Nutra-Ingredients, November 2, 2022
- 11 Nutrients, 2022;14(21) 5
- 12, 14 American Journal of Nutrition, 2011; 93(5): 997-1005
- 15 Journal of Clinical Investigation, 1975;56(5)
- 16 Journal of the International Society of Sports Nutrition, 2017, 43(14)
- 17, 23 JAMA Internal Medicine, 2018; 178(4)
- 18 Maturitas 2018;109
- 19 Journal of Aging Research, 2012;2012(316943)
- 20 Cell Reports, 2021;34(3)
- 21 Science Daily, January 20, 2021
- 22 Endocrinology and Metabolism, 2020;35(4) Section Mitochondrial dysfunction
- 24 University of Michigan School of Public Health, August 22, 2018
- 25 Diabetes Care, 2009;32(2)
- 26 Journals of Gerontology, 2018;73(8)
- 27, 28 Journal of Applied Physiology, October 1, 2005; doi.org/10.1152/japplphysiol.00061.2005