Amino Acids
now browsing by category
Common Energy Drink Ingredient May Fuel Blood Cancer
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2025/07/26/common-energy-drink-ingredient-blood-cancer.aspx
Analysis by Dr. Joseph Mercola July 26, 2025
Story at-a-glance
- Taurine, a common ingredient in energy drinks, was found to fuel the growth of leukemia cells by activating a powerful growth switch called mTOR
- In animal studies, blocking taurine’s entry into leukemia cells dramatically slowed disease progression and extended survival by up to sixfold
- Taurine supports healthy aging in animals, but too much, especially from synthetic sources, poses serious risks if cancer is already present
- The same compound that helps your cells stay young is hijacked by cancer, making the source, dose, and context important
- The safest way to use taurine is through whole foods like grass fed beef and pastured eggs; avoid overdoing supplements and skip energy drinks, especially if you’re at risk for leukemia
Energy drinks don’t just spike your adrenaline — they also feed leukemia. A study published in Nature found that taurine, a common ingredient in energy drinks and many pre-workout supplements, fuels the growth of leukemia cells.1 Researchers with the University of Rochester uncovered how this amino acid supercharges the metabolism of leukemia stem cells by activating a powerful growth pathway called mTOR.
In lab tests and animal models, supplementing taurine made leukemia worse. Taurine isn’t just a random additive. It’s naturally produced by your body and found in high concentrations in meat, fish, and dairy. It helps regulate calcium balance, support brain function and stabilize cell membranes. In healthy individuals, taurine has been shown to improve cardiovascular health, boost energy metabolism and, according to 2023 research published in Science, even extend lifespan in animals.2
So which is it? Is taurine a longevity booster or a cancer risk? The answer isn’t simple, and it comes down to how much you’re getting, from what source and whether cancer is already in the picture. To understand what’s really happening inside the body, and how something as simple as a drink additive could alter the course of a deadly disease, you need to look at what this first study uncovered.
Leukemia Stem Cells Use Taurine as Fuel to Grow and Spread
The Nature study looked at how leukemia stem cells — especially in fast-moving types like acute myeloid leukemia (AML) — survive in the body.3 Researchers found that these cancer cells don’t work alone. They get help from nearby bone marrow cells that change their environment in ways that support cancer growth. One major discovery was that taurine plays a key role in this process.
• Certain bone cells pump out extra taurine to support cancer — As leukemia gets worse, nearby bone cells, called osteolineage cells, start producing more taurine. Taurine isn’t just floating around — it’s actively pulled into the cancer cells through a special channel called the taurine–taurine transporter (TAUT) axis. This allows the leukemia cells to take in extra energy and grow faster.
• Blocking taurine’s entry into cancer cells stopped the disease from spreading — When scientists disabled the TAUT transporter in leukemia cells, the cancer slowed down dramatically. Mice with the transporter turned off lived up to six times longer. Even if taurine was still in the body, cancer cells couldn’t use it without TAUT. That shows just how important this pathway is for the cancer’s survival.
• More taurine meant faster cancer growth and earlier death — Mice that were given extra taurine had their leukemia spread faster and died up to three times sooner. Researchers also found that taurine levels were much higher in the bone marrow of mice with leukemia than in healthy ones. When they blocked the enzyme that creates taurine in bone cells, the leukemia stem cells began to die off.
• Drug-resistant leukemia cells had even more TAUT transporters — Leukemia cells that resisted chemotherapy had higher levels of TAUT, meaning they were more dependent on taurine for survival. When scientists knocked out the TAUT transporter in these cells, they stopped growing, even in lab dishes, and couldn’t survive when transferred into mice.
Taurine Flips a Growth Switch Inside Leukemia Cells
Inside the cancer cells, taurine turns on something called mTOR, which acts like a master switch for cell growth and energy use. Without taurine, this switch doesn’t turn on, and the cells can’t generate the fast energy they need. Markers of energy production dropped sharply when taurine was removed.
• Without taurine, leukemia cells lost their ability to make energy — In cells lacking TAUT, the mTOR signal dropped by threefold. Even when researchers tried to feed the cells energy shortcuts like pyruvate, which is created when your body breaks down sugar, they couldn’t fully recover. That means taurine’s role is more than just fuel — it’s a trigger for the entire energy-making process.
• Taurine sends a signal, not just nutrients — Taurine doesn’t just nourish leukemia cells — it tells them when and where to grow. It uses proteins to direct the mTOR switch to the right place in the cell. Without that signal, the growth switch stays off. Because of this, TAUT is now being studied as a new target for treating leukemia.
• This finding hasn’t yet been confirmed in humans — The study showed that taurine levels are elevated in the bone marrow of mice with leukemia, but there’s no direct evidence showing the same taurine increase in humans with acute myeloid leukemia. That means taurine’s role in human leukemia is still uncertain and needs further investigation.

Save This Article for Later – Get the PDF Now
Taurine Drops with Age, but Getting It Back Slows the Aging Process
While cancer cells hijack taurine for their own gain, healthy cells suffer when there’s not enough of it. That’s what researchers uncovered in a study published in Science.4 They wanted to know if taurine was simply a marker of aging or if it actually drives the aging process itself. What they found could change how you think about growing older.
• Taurine levels steadily decline as you age — Researchers measured taurine in mice, monkeys, and humans and saw the same trend across the board: taurine drops sharply with age. It wasn’t just a small dip — it was a consistent and measurable drop that began in middle age.
• Replacing taurine helped animals live longer and stay healthier — When middle-aged mice were given taurine supplements, they thrived. The mice lived 10% to 25% longer depending on how the data was measured. Their strength improved, their metabolism worked better, and they moved more like younger animals.
• Taurine helped the whole body, not just one part, function better — In mice, daily taurine led to stronger bones, less body fat, and more balanced immune responses. Their brains showed fewer signs of aging-related damage. In monkeys, the same pattern emerged — taurine boosted immune activity and improved mitochondrial function, which are both central to how well your body handles aging.
People with Low Taurine Were More Likely to Have Serious Health Issues
Low taurine was linked to a higher risk of obesity, high blood pressure, Type 2 diabetes, and chronic inflammation. These are the same conditions that rob people of quality of life, and in many cases, of life itself.
• Exercise was one of the few natural ways to boost taurine levels — One workout session raised taurine and its related compounds in the bloodstream. This helps explain why physical activity slows aging, because it increases a compound that repairs, regenerates, and protects your cells.5
• Taurine reversed aging at the deepest cellular level — Supplemented animals had less DNA damage, slower cell aging, and better maintenance of telomeres, the protective tips of chromosomes that shrink as you age. That means taurine helped preserve the blueprint for life inside the cell, not just the visible signs of youth on the outside.
• Taurine worked through multiple repair pathways — It supported mitochondria — the energy makers inside your cells — and calmed inflammation that damages tissues over time. It also kept stem cells functioning longer and protected immune systems from burnout. Together, these effects help explain how taurine improved health so broadly and effectively.
• Taurine extended life in complex organisms, but not in yeast — Taurine helped worms live longer, but not single-celled yeast. This suggests its antiaging effects require the presence of complex tissues and systems that communicate and repair each other — something only multicellular creatures have.
• Researchers believe taurine deficiency isn’t just a symptom of aging — it’s a cause — Replacing taurine improved multiple markers of health and longevity, which led the researchers to conclude that taurine loss is a driver of aging.
How to Use Taurine Wisely Without Feeding Disease
If you’re leaning on energy drinks or taurine supplements to push through fatigue, there’s a smarter, safer way to get your energy back. Taurine has real benefits for longevity, brain function, and cellular health, but the source and amount matter, especially if you’re facing a condition like leukemia.
In some cases, too much taurine could make things worse by feeding the disease instead of supporting your recovery. And while energy drinks look like a quick fix, they come with a long list of problems that go far beyond taurine. To protect your health:
1. Cut out energy drinks and synthetic taurine blends completely — If you’re reaching for energy drinks to boost focus or stamina, stop. These drinks are loaded with synthetic taurine and caffeine — and scientists now call them a growing public health concern. They’re linked to heart problems,6 mood issues, digestive distress, and even neurological complications.7 If your energy is low, the real fix starts by restoring healthy mitochondrial function, not flooding your system with artificial stimulants.
2. Pause taurine supplements if you’ve been diagnosed with leukemia or are at high risk — If you’re taking taurine capsules or powders, look closely at why you started. For someone with blood cancer or a strong family history, even small supplemental doses could backfire.
Leukemia cells have been shown to hijack taurine as fuel, and supplying more, especially in concentrated form, could give those cells an unfair advantage. In that case, less is more. Even if you’re healthy, don’t go overboard on taurine supplementation.
3. Focus on whole-food sources instead of artificial boosters — Taurine is naturally found in high-quality animal foods like grass fed beef, pasture-raised eggs, and shellfish. These sources give you taurine in balance with other nutrients, not in isolation. Focus on supporting your health with these natural taurine sources. Skip taurine-fortified beverages and processed products, which don’t support your body the same way.
4. Support your mitochondria, don’t overstimulate them — Instead of looking for a shortcut, think long-term. Boosting taurine should be part of a strategy to improve mitochondrial efficiency — not to mask fatigue. Regular movement, deep sleep, sunlight, and real food do more to restore energy than any supplement. Taurine works best when it’s used intentionally and in context, not on top of a lifestyle that’s already running on empty.
5. Track your response and listen to your body — Whether you’re using taurine for mood, longevity, or stamina, start small and pay attention. Use a simple log to jot down how you’re feeling each day — energy, sleep, digestion, focus. If anything feels off, back down. Your body will tell you when something isn’t working. Respect that signal.
Taurine isn’t good or bad — it’s powerful. And like anything powerful, it demands respect and careful use. The goal isn’t to chase more energy but to create the kind of balance your cells actually need.
FAQs About Taurine
Q: What is taurine, and why is it in energy drinks?
A: Taurine is an amino acid your body makes naturally, and it’s found in meat, fish, and dairy. It’s added to energy drinks and pre-workout supplements because it helps regulate energy use, brain function, and cellular stability. But in concentrated form, especially when combined with caffeine, it overstimulates the body and is harmful in certain conditions like leukemia.
Q: How is taurine linked to leukemia?
A: A study published in Nature found that leukemia stem cells hijack taurine to grow and spread, using it to activate a key growth switch called mTOR.8 In animal models, extra taurine accelerated leukemia progression, while blocking taurine’s entry into cancer cells dramatically slowed the disease and improved survival.
Q: Does that mean taurine is dangerous for everyone?
A: No. Taurine plays important roles in healthy aging and energy metabolism. Research published in Science showed that taurine levels drop with age, and supplementing it helped animals live longer and stay healthier.9 The key is using it wisely — too much, especially in synthetic or supplement form, poses risks in people with leukemia or other blood cancers.
Q: Should I avoid energy drinks with taurine?
A: Yes. Energy drinks are not a healthy source of taurine. Studies have called them a rising public health issue because they’ve been linked to heart, digestive, psychiatric, and neurological problems.10,11 If you need more energy, focus on fixing the root cause — poor sleep, stress, and mitochondrial dysfunction — instead of reaching for a taurine-loaded energy drink.
Q: What’s the safest way to get taurine?
A: Stick with taurine-rich whole foods like grass fed beef, pasture-raised eggs, and shellfish. Avoid synthetic blends and monitor how your body responds if you’re supplementing for longevity or performance. And if you’ve been diagnosed with leukemia or are at high risk, cut out taurine supplements and talk with your care team about dietary adjustments.
- 1, 3, 8 Nature May 14, 2025
- 2, 4, 5, 9 Science June 9, 2023, Vol 380, Issue 6649
- 6, 10 Heart Rhythm June 5, 2024
- 7, 11 Rev. Cardiovasc. Med. 2022, 23(3), 83
The Multifaceted Benefits of Glycine on Aging and Chronic Inflammation
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2024/10/26/health-benefits-of-glycine.aspx
Analysis by Dr. Joseph Mercola October 26, 2024

STORY AT-A-GLANCE
- Glycine, the smallest amino acid, plays an essential role in multiple physiological processes and has gained attention for its anti-inflammatory properties and ability to slow aging
- A systematic review of 50 studies, published in the journal GeroScience, found that glycine promotes healthy aging by improving cognitive functions and psychiatric symptoms
- Glycine supplementation was also shown to improve sleep quality, cognitive function and metabolic health in healthy adults, while also benefiting patients with chronic conditions
- Another recent study published in the International Journal of Molecular Sciences demonstrated glycine’s ability to reduce inflammation throughout the body, making it useful for managing chronic inflammatory conditions and supporting cellular repair mechanisms
- Practical ways to boost glycine intake include consuming collagen-rich foods and taking glycine powder; more tips are included below
Glycine is the simplest and smallest amino acid in your body, but don’t let its size fool you. This little molecule plays a big role in multiple physiological processes, from building proteins to supporting your immune system. A growing body of research also shows that glycine plays a far more significant role than previously thought.
Recently, glycine has gained attention in the scientific community for its anti-inflammatory properties and ability to slow down the aging process. This dual action makes glycine a compelling subject for those seeking natural ways to promote overall well-being and longevity, and transforms how we approach chronic diseases, aging and overall health maintenance.
Glycine’s Impact on Aging and Longevity
As the global population grows older, the need for safe interventions to maintain vitality becomes increasingly important. This prompted researchers to conduct a systematic review of 50 total studies to determine the effect of glycine administration on various physiological systems as we age. Published in GeroScience, the researchers noted:1
“Most studies (42 over 50) were randomized controlled trials (RCT), of which half were parallel-group trials. The majority of studies (41 over 50) reported oral glycine ingestion as the mode of delivery. Eighteen out of 50 studies were in healthy populations, 34 [out of] 50 in diseased populations and 2 [out of] 50 contained both healthy and diseased populations.
The mean or median age ranged from 21.5 to 41.4 years for healthy populations and 29.5 to 67 years for diseased populations. Glycine was administered for a period of one day (single bolus) to 14 days in healthy populations and up to 4 months in diseased populations.”
Their findings showed that glycine had the most pronounced effect on the nervous system, particularly in patients with psychiatric conditions. For instance, schizophrenic patients experienced notable improvements in psychiatric symptoms, cognition and sleep after long-term glycine supplementation.
This is largely attributed to glycine’s role as a co-agonist at the N-methyl-D-aspartate (NMDA) receptor, which is vital for neural communication and plasticity. The activation of NMDA receptors by glycine has been shown to enhance cognitive and neurological functions.
“Schizophrenia is hypothesized to result from the hypofunctioning of NMDA receptors. Several reports cited herein have particularly underscored the … effect of glycine on the NMDA receptor in eliciting positive neurological outcomes.”2
In addition to its effects on the nervous system, glycine demonstrated significant results in other physiological systems. In healthy populations, studies reported improved insulin responses, indicating benefits for metabolic health. For patients with chronic conditions, such as those undergoing hemodialysis, glycine supplementation improved handgrip strength and fat-free mass index, positively impacting muscle function and body composition.3
Glycine Improves Sleep and Cognitive Function in Healthy Adults
The GeroScience review4 also observed significant benefits of glycine supplementation on sleep quality and cognitive function in healthy individuals. Sleep disturbances become a common concern as you age, and glycine appears to offer a promising solution.
The researchers observed that those who took glycine before bedtime fell asleep faster and reported better sleep quality. They also experienced better cognitive function the next day. According to the authors:5
“Improved sleep quality, alertness and cognition, and decreased fatigue were observed in three populations receiving 3 grams per day oral administration of glycine 30 minutes to one hour before bedtime over two to four days.”
The study also highlighted glycine’s role in regulating body temperature, which is important for initiating and maintaining sleep. Glycine administration was found to promote hypothermia and vasodilation through its action on NMDA receptors in the suprachiasmatic nucleus (SCN), the master circadian pacemaker.6
Although these findings suggest that glycine may help maintain brain function as we age, the results were based on smaller studies, and the researchers acknowledged the need for larger, long-term trials to confirm these effects. Nevertheless, glycine’s ability to improve daytime cognitive function and promote restful sleep highlights it as a simple yet powerful tool for supporting healthy aging.7

Save This Article for Later – Get the PDF Now
Glycine — The Natural Solution to Chronic Inflammation
The quest for effective anti-inflammatory compounds has intensified in recent years, stemming from the alarming increase in chronic inflammatory conditions plaguing modern society. Traditional medications often come with unwanted side effects, leading researchers to explore nutritional approaches for managing inflammation. Glycine has emerged as a candidate in this search.
A paper published in the International Journal of Molecular Sciences,8 titled “Glycine: The Smallest Anti-Inflammatory Micronutrient,” highlights glycine’s ability to reduce inflammation throughout the body. These findings are important because inflammation is at the root of many chronic diseases. According to the authors:9
“Glycine could modulate the low-grade inflammatory process through pathways that involve some of its targets that have already been identified in different cells … For decades, glycine has been proposed as an anti-inflammatory agent and used as a therapeutic nutrient to treat inflammation related to diseases such as arthritis, gastric ulcers, melanoma, alcoholic liver disease and endotoxic shock.”
The study also references various clinical trials that underscore glycine’s therapeutic benefits. For instance, in patients with rheumatoid arthritis, glycine supplementation led to reduced joint pain and inflammation, with researchers noting that glycine decreased the production of inflammatory molecules in the body, leading to improved symptoms.10
Alcoholic liver disease is another condition where glycine shows benefits. In one clinical trial, patients with alcohol-induced liver damage who received glycine supplements saw improvements in liver function tests. Glycine appeared to protect liver cells from the harmful effects of alcohol, reducing inflammation and supporting the liver’s natural detoxification processes.11
Glycine’s impact extends to the nervous system, where it plays a neuroprotective role. By suppressing the activation of proinflammatory microglia cells, it helps prevent neuroinflammation.12 This protective effect on brain cells is beneficial for cognitive health as we age.
Metabolic health also benefits from glycine. In a clinical trial, individuals with metabolic syndrome who supplemented with glycine for three months saw improvements in several key health markers, including reduced inflammation, better insulin sensitivity and improved blood lipid profiles.13
Glycine’s influence on cellular repair mechanisms is particularly noteworthy. Glycine supports the production of glutathione, one of the body’s most powerful antioxidants.14 This boost in antioxidant capacity helps protect cells from damage caused by everyday stressors, slowing the aging process at a cellular level.
How Glycine Works Its Magic in Your Body
Glycine is efficient at calming down inflammation. According to the study in the International Journal of Molecular Sciences,15 it does this by putting the brakes on some important signals in your body that usually ramp up inflammation. Studies show it reduces the production of proinflammatory molecules like TNF-α and interleukin-6 while increasing anti-inflammatory factors such as IL-10. It also inhibits NF-κB from getting activated.
Glycine also binds to specific receptors in your cells called glycine-gated chloride channels.16 When these channels open, they allow chloride ions to flow into the cell, temporarily changing it to a negative electrical charge. This process, known as hyperpolarization, calms down overactive immune cells and nerve cells, which in turn is helpful for fighting inflammation and protecting your brain.
Glycine also aids your body in the production of an important antioxidant called glutathione, which protects your cells from damage.17 By helping your body make more glutathione, glycine is basically boosting your cellular defenses. Additionally, glycine influences the beneficial organisms living in your gut, known as your microbiome.18
A healthy gut microbiome is crucial for your overall health, from your immune system to your mood. So, to sum it up, here’s a quick list of how glycine boosts your health:
- Calms inflammation signals
- Inhibits overactive immune and nerve cells
- Boosts the production of protective antioxidants
- Helps balance your gut microbiome
Boosting Your Glycine Levels — Simple Strategies for Better Health
Here are some practical ways to increase your intake of this powerful amino acid:
- Eat more collagen- and gelatin-rich food — Glycine makes up nearly one-third of collagen and gelatin. Some good sources include homemade bone broth made with bones and connective tissue from grass fed, organically raised animals, and chicken broth made from organic chicken feet. The claws are particularly rich in collagen.
- Consider glycine supplements — Pure glycine is available in powder form, which has a mildly sweet flavor, offering an affordable, convenient way to boost your intake.
- Pair glycine with complementary nutrients — Vitamin C aids collagen synthesis, working synergistically with glycine. Foods rich in vitamin C include citrus fruits, bell peppers and leafy greens.
- Time your glycine intake strategically — For sleep benefits, take glycine about an hour before bedtime. If using glycine to support exercise recovery, consume it shortly after your workout.
- Support your body’s natural glycine production — Eating a varied diet rich in protein provides the building blocks your body needs to make glycine. Animal-based foods like lean meats and organic, pastured eggs from chickens fed a low-PUFA diet will raise your glycine levels, as they contain taurine, which increases glycine.
Remember, glycine is just one piece of the health puzzle. Combine these strategies with an anti-inflammatory diet, regular exercise and stress management techniques for maximum benefit.
- 1, 2, 3, 4, 5, 6, 7 GeroScience (2024) 46:219–239, doi: 10.1007/s11357-023-00970-8
- 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Int J Mol Sci. 2023 Jul 8;24(14):11236. doi: 10.3390/ijms241411236
Higher Taurine Intake Correlates With Some Measures of Strength in Middle Age
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2024/05/31/taurine-may-support-muscle-strength-middle-age.aspx
Analysis by Dr. Joseph Mercola May 31, 2024

STORY AT-A-GLANCE
- Taurine is an amino acid found in animal foods such as seafood, grass fed red meat, dairy products and pastured eggs
- Taurine is considered a “conditionally essential,” or semi-essential, amino acid because, while your body can naturally produce it, supplementation might be necessary under certain conditions
- Research involving Japanese adults suggests higher taurine intake may protect muscle strength in middle age and beyond
- Higher taurine intake was linked to a significant increase in knee extension muscle strength over eight years
- Taurine levels are thought to play a key role in aging and longevity, however levels typically decline with age
Taurine is a type of amino acid, which are the building blocks of proteins. Unlike many other amino acids, taurine is not used to build proteins but rather plays several other critical roles in the body, such as supporting nerve growth, producing bile salts and helping with digestion and maintaining proper hydration.1
Taurine is considered a “conditionally essential,” or semi-essential, amino acid because, while your body can naturally produce it, supplementation might be necessary under certain conditions, such as in infants or in people with specific medical conditions.
Taurine levels, however, decline with age2 and are thought to play a key role in aging and longevity. In fact, research involving Japanese adults suggests higher taurine intake may protect muscle strength in middle age and beyond.3
Higher Taurine Intake May Support Muscle Strength in Middle Age
The study was conducted as part of the National Institute for Longevity Sciences-Longitudinal Study of Aging (NILS-LSA) in Japan. Participants, all aged 40 years or older, provided dietary data at the beginning of the study and underwent physical fitness assessments at the start and eight years later.
The researchers specifically investigated how taurine intake affected changes in four measures of physical fitness: knee extension muscle strength, flexibility (sit-and-reach test), balance (one-leg standing with eyes closed) and walking speed. Adjustments were made in the analysis for factors such as sex, age, body measurements, educational level, health status, smoking, depressive symptoms and medical history to isolate the effects of taurine.
The average daily taurine intake among the study participants was 207.5 milligrams (mg). However, higher taurine intake was linked to a significant increase in knee extension muscle strength over eight years. According to the study:4
“Knee extension muscle strength is an indicator of lower limb muscle strength, which is directly related to the ability to perform activities of daily living, such as walking and standing. Muscle strength is influenced by the muscle cross-sectional area and fast/slow muscle fiber ratio. Aging leads to a decrease in muscle cross-sectional area and fast-muscle fiber size, leading to muscle weakness.”
This positive association was particularly notable in participants aged 65 years and older, where higher taurine intake correlated with a slower decline in muscle strength — although taurine intake did not show a significant relationship with the other assessed fitness parameters of flexibility, balance and walking speed.
The research suggests that taurine intake from the diet could play a crucial role in preserving muscle strength among older adults, marking the first research to link dietary taurine with muscle strength maintenance over time. It’s also possible, however, that taurine may serve as a marker for intake of other beneficial compounds in the diet. As noted by Fight Aging!:5
“In the context of recent studies on taurine supplementation, [this] … open access paper seemed interesting. The authors report on correlations between taurine intake in the normal diet with a few measures of fitness and muscle strength in middle-aged individuals. Human studies of taurine supplementation require a dose in the range of 1.5-6.0 grams per day to remove the 50% loss in circulating taurine.
This supplement dose is the human equivalent extrapolated from the effective doses in mice and non-human primates. Here, dietary intake of taurine in the study participants was estimated to be ~200 milligrams per day, which is actually higher than previously reported averages, particularly for vegetarians.
Given that, one might argue that taurine levels in the diet are a proxy for the influence of some other better-studied aspect of dietary choices on long-term health, such as overall protein intake.”
Taurine Helps Keep Aging Muscles Strong
Taurine has been found to play a significant role in countering the effects of aging on muscle regeneration, the researchers explained.6 This suggests that taurine not only supports normal muscle function but may also be crucial in maintaining muscle strength as people age.7
Long-term consumption of taurine could enhance its levels in muscle tissues, helping regulate the crucial flow of calcium ions that are essential for muscle contractions. This process could be key to maintaining muscle strength and overall physical health in middle-aged and older adults.8 Further, animal studies have revealed that taurine plays a role in how muscles function, affecting:9
- Muscle performance — Taurine helps muscles contract more effectively by managing the flow of calcium ions within muscle cells. This is important because calcium ions are key to muscle contractions.
- Muscle relaxation — When there’s not enough taurine, muscles may not relax as smoothly after contracting.
- Aging and muscle health — In older rats, adding taurine to their diet increased its presence in their muscles, which improved muscle function. Conversely, animals that couldn’t transport taurine properly showed signs of faster aging and had shorter lifespans. Their muscles also aged quicker both in appearance and function.10
- Heart health — Low taurine levels can make the heart prone to fibrosis, a condition where the heart becomes stiffer as you age.
Taurine also acts as an antioxidant and anti-inflammatory agent that may be useful in warding off sarcopenia, an age-related condition characterized by the loss of muscle mass and function. In a study involving older mice, taurine supplementation counteracted the effects of aging in skeletal muscle.11
Specifically, taurine helped improve muscle regeneration after injury by reducing inflammation and preserving muscle fiber integrity. It also reduced oxidative stress in aged muscles by maintaining cellular redox balance.

Save This Article for Later – Get the PDF Now
Is Taurine Deficiency a Driver of Aging?
Of the amino acids, taurine is the most abundant source of sulfur and is required for a wide range of physiological processes, including the healthy function of your immune system, nervous system,12 metabolism and digestion — but that’s not all.
According to research published in the June 2023 issue of the journal Science, taurine also appears to play an important role in longevity and healthy aging.13 According to the editor’s summary of the study:14
“Supplementation with taurine slowed key markers of aging such as increased DNA damage, telomerase deficiency, impaired mitochondrial function, and cellular senescence. Loss of taurine in humans was associated with aging-related diseases, and concentrations of taurine and its metabolites increased in response to exercise. Taurine supplementation improved life span in mice and health span in monkeys.”
For the study, researchers gave taurine supplements to middle-aged mice daily. Remarkably, both male and female mice that received taurine lived longer than those that didn’t, with their life spans increasing by about 10% to 12% and their life expectancy at 28 months rising by 18% to 25%.15
But extending life isn’t enough; the quality of that extended life is also crucial. The study found that taurine not only helped the mice live longer but also kept them healthier for longer. The supplemented mice showed improved functions in critical areas such as bones, muscles, pancreas, brain, fat, gut and the immune system, effectively increasing their health span, or their period of healthy living.
Similar results were observed in monkeys and even extended to other species, like worms. Further investigation revealed that taurine supplementation reduced common signs of aging. It helped decrease cell aging, protected against damage to the ends of chromosomes, improved mitochondrial function, reduced DNA damage and lowered inflammation.16
Moreover, in humans, lower levels of taurine and related compounds were linked with several health issues, including obesity, high blood pressure, inflammation and diabetes. Interestingly, exercise was found to increase taurine levels in the blood, which may help explain some of the antiaging benefits of physical activity.
Overall, taurine supplementation could be a promising way to not only extend life span but also improve quality of life as we age, by mitigating various biological signs of aging. “This identifies taurine deficiency as a driver of aging,” the researchers concluded.17
Taurine Deficiency Linked to Chronic Diseases
In addition to playing a key role in longevity, a deficiency of taurine may contribute to chronic disease. Research suggests people with lower blood levels of taurine have increased risk of several chronic and/or degenerative diseases, including:18,19,20
| Obesity | Diabetes |
| Insulin resistance | Liver disease |
| High blood pressure | Systemic inflammation |
| Retinal degeneration | Heart disease |
| Immune dysfunction | Muscle wasting |
Patients suffering from heart failure also tend to be deficient in taurine, which is thought to be related to its ability to improve mitochondrial function and energy metabolism. Restoring taurine levels in these patients has been shown to improve the contractile function of their hearts.21 Stroke victims may also benefit from taurine, which is able to cross the blood-brain barrier and is beneficial for the central nervous system.22
Taurine deficiency is also associated with endoplasmic reticulum stress,23 a major contributor to prion diseases. Taurine is also thought to be important for proper protein folding. As such, taurine may also be an important aid in the treatment of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Signs of taurine deficiency are varied due to taurine’s many biological effects. Common symptoms include:24,25,26
- Fatigue and low energy, as taurine is involved in energy production
- Muscle cramps, muscle weakness, muscle wasting/atrophy and poor exercise performance, as taurine is essential for muscle health and function
- Increased oxidative stress and systemic inflammation, which contributes to and is a hallmark of most chronic diseases
- Impaired immune function, as taurine is involved in immune cell function and the regulation of inflammation
- Vision problems associated with retinal degeneration, as taurine is essential for development and maintenance of the cells in your retina
Food Sources of Taurine
Taurine is found in animal foods such as seafood, red meat, poultry and dairy products, and it’s always best to get your nutrients from your diet.
If you’re a vegan, however, you may want to consider a high-quality taurine supplement, as you’re not getting any from the foods you eat. While your body can synthesize some taurine, it’s not going to be sufficient in the long run, especially as you get older and your body’s ability to synthesize it diminishes.
- 1 Cleveland Clinic October 1, 2023
- 2, 13, 14, 15, 16, 17 Science June 9, 2023
- 3, 4 Front. Nutr., 20 March 2024
- 5 Fight Aging! April 16, 2024
- 6, 8, 9 Front. Nutr., 20 March 2024, Discussion
- 7 J Pharmacol Exp Ther. 1998 Sep;286(3):1183-90
- 10 PLoS One. 2014 Sep 17;9(9):e107409. doi: 10.1371/journal.pone.0107409. eCollection 2014
- 11 Curr Protein Pept Sci. 2018 Jul; 19(7): 673–680
- 12 Journal of Biomedical Science August 24, 2010; 17 Article No. S1
- 18, 25 Science June 9; 380(6649) DOI: 10.1126/science abn9257
- 19, 26 Biomolecules & Therapeutics 2018; 26(3): 225-241
- 20 Twitter Dr. Eashwarran Kohilathas April 7, 2023
- 21 American Heart Journal June 2002; 143(6): 1092-1100
- 22 Brain Sciences June 2013; 3(2): 877-907
- 23 Adv Exp Med Biol 2015; 803: 481-487
- 24 Biomol Ther (Seoul). 2018 May; 26(3): 225–241
Glycine Reverses Aging in Cells
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2023/10/02/glycine-reverses-aging-in-cells.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola Fact Checked October 02, 2023
STORY AT-A-GLANCE
- Collagen is one-third of the protein in your body and 28% of it is made up of the amino acid glycine. One study estimates most people are about 10 grams short of what their bodies need for all metabolic uses on a daily basis
- Mounting research suggests glycine may play an important role in the aging process. It’s been shown to extend lifespan in worms, mice and rats, and improve health in mammalian models of age-related disease
- Glycine is a precursor to glutathione, a powerful endogenous antioxidant that declines with age, and the lack of glutathione in older adults may be an element that drives the oxidative stress and mitochondrial dysfunction that lead to age-related degeneration
- Glycine also acts as a neurotransmitter and may play an important role in depression, neuroinflammation, neurodegeneration and cognitive decline
- Glycine may even be responsible for the epigenetic regulation that drives the aging process. Regulation of the aging process in your mitochondria appears to be ruled by two genes that regulate glycine production in the mitochondria. Adding glycine to the culture medium of fibroblast cells taken from 97-year old people restored the cells’ respiratory function, which suggests that glycine treatment can reverse the age-associated respiration defects in human fibroblasts
Collagen — which provides structural support and strength to your tissues1,2,3 — accounts for about 30% of the total protein in your body. Twenty-eight percent of collagen, in turn, is made up of the amino acid glycine.4
Glycine, proline and hydroxyproline5 are the raw materials for connective tissue, but the benefits of glycine go far beyond connective tissue health. In fact, mounting research suggests glycine may play an important role in the aging process.
While your body does make glycine, endogenous production decreases with age, and if you only eat red meat, and rarely or never consume foods made with collagen-rich connective tissue, you’re likely not getting enough from your diet either. The chart below details the amino acid ratios of gelatin/collagen versus red meat (beef). As you can see, gelatin/collagen contains vastly more glycine than beef.
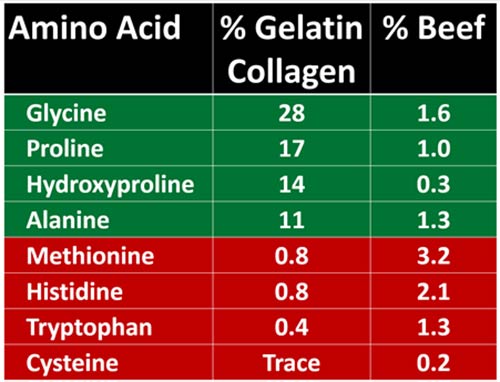
Glycine Has Many Antiaging Benefits
Glycine has been shown to extend lifespan in worms, mice and rats, and to improve health in mammalian models of age-related disease.6 In some animal studies, eating a diet containing 8% to 12% glycine increased the median lifespan by as much as 28.4%.7
As explained by Siim Land, author of “Metabolic Autophagy: Practice Intermittent Fasting and Resistance Training to Build Muscle and Promote Longevity,” in the video above, glycine induces autophagy (a “self-eating” process in which your body digests damaged cells) and mimics the longevity benefits of methionine restriction.8 Both of these effects are related to an enzyme called glycine N-methyltransferase (GNMT).
Glycine is a receptor for GNMT, and the GNMT converts glycine to sarcosine, a metabolite that induces autophagy. GNMT also plays a role in methionine clearance.9 Methionine is involved in cancer cell growth and metabolism, and restricting methionine has been shown to:10
- Inhibit cancer cell growth
- Extend lifespan
- Lower levels of insulin, glucose and insulin-like growth factor 1 (IGF-1)
- Reduce liver damage after exposure to dangerous amounts of acetaminophen
- Reduce frailty
Even intermittently restricting methionine leads to benefits like improved glucose homeostasis, reduced obesity and protection against fatty liver disease.11 Glycine is also a precursor to glutathione, a powerful endogenous antioxidant that declines with age.
The lack of glutathione in older adults may be an element that drives the oxidative stress and mitochondrial dysfunction that leads to age-related degeneration. Glycine also acts as a neurotransmitter12 and may play an important role in depression.13 It’s also been shown to alleviate neuroinflammation and protect against cognitive deficits in mice with neurodegeneration.14
Glycine Reverses Aging in Human Cells
According to previous research,15 glycine may even be responsible for the epigenetic regulation that drives the aging process as a whole. As reported by Science Daily in 2015,16 contrary to popular belief, the aging process in your mitochondria may not be controlled by DNA mutations after all, but rather by epigenetic regulation.
This epigenetic regulation appears to be ruled by two genes (CGAT and SHMT2) that regulate glycine production in the mitochondria. By altering the regulation of these genes, the researchers were able to either induce defects or restore mitochondrial function in human fibroblast cell lines.
Remarkably, simply adding glycine to the culture medium of fibroblast cells taken from 97-year-old people restored the cells’ respiratory function, which “suggests that glycine treatment can reverse the age-associated respiration defects in the elderly human fibroblasts.” As such, glycine supplementation could potentially give the elderly “a new lease on life.”
According to one study, most people are about 10 grams short of what their bodies need for all metabolic uses on a daily basis.

Download this Article Before it Disappears
Glycine Is Required in Daily Collagen Turnover
Making up 28% of your body’s collagen, glycine is, of course, also required for optimal collagen synthesis. As explained by Land in the featured video:17
“Glycine also has a very important role in antiaging directly by helping to reduce wrinkles, and collagen synthesis … The less collagen or glycine you consume, the slower your collagen turnover is.
Slow collagen turnover increases the damage that occurs to your collagen, such as glycation and oxidation, and reduces collagen deposition into tissues.
Collagen … makes up your hair, teeth, skin, nails, organs, arteries, cartilage, bones, tendons and ligaments. Collagen is literally the glue that holds you together. So, making sure that you preserve your collagen is very important for slowing down aging, especially when it comes to wrinkles.
Starting at the age of 20 you lose just under 10% of your skin’s collagen content every decade. So, by the time you’re 75 years old you would have lost 50% of your skin’s collagen content …
[U]p until very recently it was thought that the collagen turnover was very slow, and it only happened over the course of many years — over the entire lifespan. However, recently it was shown that college turnover happens every day and is part of your daily protein turnover.”
Glycine Protects Against Age-Related Disease
Glycine also helps mitigate chronic disease and disability, thereby increasing your health span. As reported in a 2023 scientific review, glycine has been shown to:18
| Suppress tumor growth in mice with melanoma19 | Decrease fasting glucose, insulin, triglyceride and IGF-1 in male rats20 |
| Preserve muscle mass and reduce inflammatory markers in mice with cancer cachexia21 | Improve endothelial function in older rats22 |
| Reduce weight gain and improve bone mineral density in a mouse model designed to mimic postmenopausal bone loss23 | Protect against cardiac hypertrophy24 |
| Alleviate neuroinflammation and protect against cognitive deficits in mice with neurodegeneration25 |
Human trials also confirm that glycine is protective against a range of chronic diseases. As noted by Land:
“The benefits of glycine generally have to do with improving the blood sugar levels, fasting insulin levels, triglycerides, even lowering the demand for sleep, improving brain function and health, helping with just overall aspects of vitality.”
Other Health Benefits of Glycine
Other health benefits of glycine include:
- Improved sleep26
- Reduced inflammation and oxidative damage, as glycine inhibits the consumption of nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is used as a reductive reservoir of electrons to recharge antioxidants once they become oxidized
- Reduced stress27
- Improved wound healing28
- Improved gut health29
In his article “Gelatin, Stress, Longevity,”30 the late biologist, Ray Peat, reviewed a long list of health conditions that can be prevented or alleviated by glycine supplementation and/or increased consumption of collagen or gelatin. These include:31
| Fibrosis | Most bleeding problems, including nosebleeds, excessive menstrual bleeding, bleeding ulcers, hemorrhoids and stroke. According to Peat, glycine, taken shortly after a stroke, limits the damage and accelerates recovery |
| Epilepsy, by stabilizing nerves and raising the amount of stimulation required to activate nerves | Multiple sclerosis (MS), thanks to its antispastic effects |
| Any condition involving excess prolactin, serotonin and/or cortisol, including autism, postpartum and premenstrual problems, Cushing’s disease, diabetes, and impotence | Fatigue |
| Muscular dystrophy and myasthenia gravis | Metabolic disorders32 |
| Nonalcoholic fatty liver disease (NAFLD)33 | Schizophrenia34 |
How to Optimize Your Glycine Intake
When it comes to optimizing your glycine intake, you have several basic options:
1. Eat more collagen or gelatin-rich foods, as glycine makes up nearly one-third of collagen and gelatin. Examples include homemade bone broth made with bones and connective tissue from grass fed, organically raised animals, and chicken broth made from organic chicken feet. The claws are particularly rich in collagen.35
Indirectly, animal foods such as seafood, red meat, poultry and dairy products will also raise your glycine level, as these foods contain taurine, which increases glycine.36
2. Take a high-quality collagen or gelatin supplement.
3. Take a glycine supplement. Pure glycine is available in powder form and tends to be very affordable and easy to take, as it has a mildly sweet flavor.
Considering its many health benefits, making sure you get enough glycine in your diet can go a long way toward improving your health and life span. There’s no established daily requirement or upper limit of glycine currently, so it’s hard to make specific recommendations.
That said, doses of 3 to 5 grams have been shown to improve sleep.37 One study38 estimated that most people are about 10 grams short of what their bodies need for all metabolic uses on a daily basis,39 and in a study of people with metabolic syndrome, 15 grams of glycine a day for three months reduced oxidative stress and improved systolic blood pressure.40 That should give you an approximate idea.
- 1 Bone 2010 Mar;46(3):827-3
- 2 PLoS One 2014 Jun 13;9(6):e99920
- 3 J Agric Food Chem. 2010 Jan 27;58(2):835-41
- 4 Molecular Cell Biology, 4th Edition, Section 22.3. 2000
- 5 Amino Acids 2018 Jan;50(1):29-38
- 6 Ageing Research Reviews March 31, 2023, Highlights
- 7 Ageing Research Reviews March 31, 2023, Table 1
- 8, 9, 10 Ageing Research Reviews March 31, 2023
- 11, 18 Ageing Research Reviews March 31, 2023, Table 2
- 12 Ageing Research Reviews March 31, 2023, Intro
- 13 Medical News Today April 4, 2023
- 14, 25 Journal of Neuroinflammation October 15, 2020; 17, Article number 303
- 15 Scientific Reports 2015; 5 Article number 10434
- 16 Science Daily May 26, 2015
- 17 YouTube, Siim Land, Glycine Longevity Benefits Are Amazing – New Study Confirms April 5, 2023, 5:49, 7:31
- 19 Carcinogenesis, Volume 20, Issue 5, May 1999, Pages 793–798
- 20 The FASEB Journal April 1, 2011
- 21 Clinical Nutrition June 2014, Volume 33, Issue 3, Pages 448-458
- 22 Canadian Journal of Physiology and Pharmacology March 4, 2015; 93(6)
- 23 Amino Acids 2016; 48: 791–800
- 24 Biochemical Pharmacology January 1, 2017; 123: 40-51
- 26 J Pharmacol Sci 2012; 118: 145 – 148 (PDF)
- 27, 28, 30, 31 RayPeat.com Gelatin Stress and Longevity
- 29 Am J Physiol 1982 February;242(2):G85-8
- 32, 33 Nutrients June 2019, 11(6): 1356
- 34, 37 Examine Glycine
- 35 TheHealthBenefitsOf.com 10 Chicken Feet Health Benefits
- 36 Anxiety Medication, Taurine
- 38 J Biosci December 2009; 34(6): 853-872
- 39 Supplements Self Decode Glycine
- 40 Canadian Journal of Physiology and Pharmacology June 17, 2013
Why Collagen Is a Proven Necessity
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2023/09/04/why-collagen-is-a-proven-necessity.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola Fact Checked September 04, 2023

STORY AT-A-GLANCE
- Collagen is the most common and abundant of your body’s proteins, accounting for about 30% of the total protein in your body. One of its primary purposes is to provide structural scaffolding to allow tissues to stretch and flex while maintaining tissue integrity
- Collagen is found in your skin, connective tissues like tendons, ligaments, cartilage and fascia, your bones, organs, blood vessels, musculoskeletal system, hair and nails
- The loss of collagen that occurs with age is the primary reason for wrinkles, dry sagging skin and lackluster hair. You can maintain a more youthful appearance by making sure you’re getting plenty of collagen and/or gelatin in your diet
- Collagen is also crucial for bone health and recovery from soft tissue injuries, and can help improve sleep, reduce joint pain, improve gut health, glucose tolerance and blood pressure, reduce cardiovascular damage, lower your risk of osteoporosis, and lower inflammation and oxidative damage
- The primary amino acids in collagen — glycine, proline and hydroxyproline — make up the matrix of connective tissue. Beef contains very little of these amino acids, so eating only muscle meat will not provide enough amino acids to allow you to build strong connective tissue and maintain bone strength
Collagen is the most common and abundant of your body’s proteins, accounting for about 30% of the total protein in your body. It’s found in your skin, connective tissues like tendons, ligaments, cartilage and fascia, your bones, organs, blood vessels, musculoskeletal system and even your hair and nails. One of its primary purposes is to provide structural scaffolding for tissues to allow them to stretch and flex while maintaining tissue integrity.
Collagen Helps Maintain a More Youthful Appearance
The loss of collagen that occurs with age is the primary reason for wrinkles, dry sagging skin and lackluster hair. When your collagen level is high, your skin will tend to be soft, smooth and firm, because the collagen allows skin cells to continuously repair and renew themselves.
By the time you reach your 60s, you have about half the collagen you did in your youth, and once you enter your 80s, you have about four times less, hence the radical changes in your skin.
If you’re vegetarian or vegan, signs of skin aging may be more pronounced for the simple fact that you don’t eat collagen-rich foods on a regular basis. Foods like fish, bone broth and organic, pastured chicken and eggs are all natural sources of collagen. Many vegetarians and vegans also shun collagen supplements because they’re made from animal sources.
When it comes to skin health, it’s important to realize that topically applied collagen cannot cross into deeper skin layers, so most collagen-containing skin creams are likely a waste of money. To really make a difference, you need to tackle the problem from the inside-out. The good news is you can maintain a more youthful appearance by making sure you’re getting plenty of collagen and/or gelatin in your diet.1,2,3
Collagen Supports Optimal Health in Many Ways
The benefits of collagen certainly don’t end there, though. Collagen is also crucial for bone health,4,5,6 and will dictate how well and how rapidly you’ll recover from soft tissue injuries. Collagen can also help:
| Improve your sleep7 | Reduce joint pain and stiffness,8 including osteoarthritis pain9 |
| Improve digestion10 and gut health by keeping your gut lining healthy11 | Improve glucose tolerance12 |
| Improve blood pressure13 | Reduce cardiovascular damage14 |
It can also help lower your risk of osteoporosis (brittle bone). According to ABC15 Health Insider Dr. Shad Marvasti, adding 10 to 15 grams of collagen a day has been shown to improve bone health in as little as eight weeks.15
Thanks to its high glycine content, collagen also helps reduce inflammation and oxidative damage, which are hallmarks of most chronic diseases. The amino acid glycine, which makes up 28% of collagen, does this by inhibiting the consumption of nicotinamide adenine dinucleotide phosphate (NADPH), which acts as a reductive reservoir of electrons to recharge antioxidants once they become oxidized.

Download this Article Before it Disappears
Collagen Is Required for Strong Connective Tissue and Bone
Connective tissues such as tendons, ligaments, cartilage and fascia also tend to get weaker and less elastic with age, making you more prone to injuries that can take a long time to heal.
Connective tissue injuries are also problematic because there’s very little blood supply in connective tissue, which slows recovery. While a muscle injury is relatively easy to recover from, connective tissue requires collagen to heal, as glycine,16 proline and hydroxyproline17 are the raw materials that make up the matrix of connective tissue.
Beef contains very little of the amino acids required for connective tissue health. So, beef alone will not allow you to build strong connective tissue and maintain bone strength.
Interestingly, research suggests your body will selectively take collagen into the areas that are stressed and need it most. We discussed this in my 2018 interview with Mark Sisson, a former elite endurance athlete.
As you can see in the chart below, beef contains very little of the amino acids required for connective tissue health. So, beef alone will not allow you to build strong connective tissue and maintain bone strength.
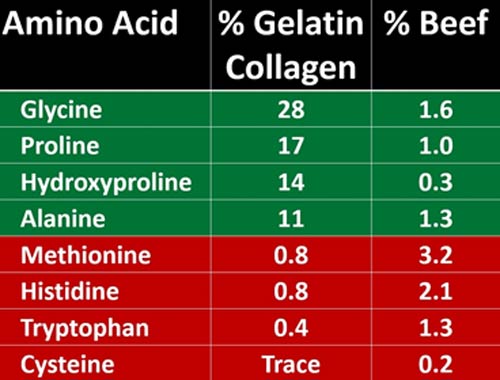
If you are only eating muscle meats without the connective tissue, you will get the amino acids in the right column, which simply does not provide your body with the amino acids it requires to build collagen. Admittedly, these missing amino acids are not essential so your body can make them, but it will waste loads of energy in doing that.
Bone Formation
Bone is created when collagen fibrils mineralize together with carbonated hydroxyapatite (calcium apatite). Combined, they form a hybrid material that is very strong yet flexible.
What’s more, as other minerals (such as strontium- and calcium-based minerals) are deposited inside the collagen, it causes a reaction that triggers the collagen fibrils to contract. This stress generates a mineral-collagen composite material composed of high-tensile fibers with properties reminiscent of reinforced concrete.18,19
This explains why tendons have the tensile strength of wire ropes and why healthy bones are so hard yet not brittle.20 As with connective tissue, the key to strong and flexible bone is collagen, and if you’re not trying to maintain a healthy intake, your bones will become increasingly brittle and less strong with age, ultimately resulting in osteoporosis.
Collagen for Life Extension and Disease Prevention
If you are getting the majority of your protein from muscle meats you will be getting high amounts of the amino acids that are in red, which are very low in collagen and gelatin. Why is this important? Because these are the very amino acids that, when consumed in excess, have been highly correlated with decreased longevity.
Your collagen intake may also impact your longevity and overall disease risk. As reported by the late Ray Peat, a biologist who specialized in bioenergetic medicine,21 life extension studies have shown that restricting tryptophan or cysteine alone produces greater life extension than calorie restriction, which is rather remarkable.
Referring to the chart above, you can see that beef contains more than three times the amount of tryptophan compared to collagen. Peat also argued that collagen, the cooked form (gelatin) in particular, has a long history of use for disease prevention. Modern medicine has simply chosen to overlook or forget all of that. In his archived article “Gelatin, Stress, Longevity,” Peat explained:22
“Both tryptophan and cysteine inhibit thyroid function and mitochondrial energy production, and have other effects that decrease the ability to withstand stress. Tryptophan is the precursor to serotonin, which causes inflammation, immunodepression, and generally the same changes seen in aging …
[G]elatin is a protein which contains no tryptophan, and only small amounts of cysteine … Using gelatin as a major dietary protein is an easy way to restrict the amino acids that are associated with many of the problems of aging …
When cells are stressed, they form extra collagen, but they can also dissolve it, to allow for tissue remodeling and growth … When collagen is broken down, it releases factors that promote wound healing and suppress tumor invasiveness.
Glycine itself is one of the factors promoting wound healing and tumor inhibition. It has a wide range of antitumor actions, including the inhibition of new blood vessel formation (angiogenesis), and it has shown protective activity in liver cancer and melanoma …
When we eat animal proteins in the traditional ways (for example, eating fish head soup … ‘head-cheese’ … and chicken-foot soup …), we assimilate a large amount of glycine and gelatin …
When only the muscle meats are eaten, the amino acid balance entering our blood stream is the same as that produced by extreme stress, when cortisol excess causes our muscles to be broken down to provide energy and material for repair.
The formation of serotonin is increased by the excess tryptophan in muscle, and serotonin stimulates the formation of more cortisol, while the tryptophan itself, along with the excess muscle-derived cysteine, suppresses the thyroid function …
The range of injuries produced by an excess of tryptophan and serotonin seems to be prevented or corrected by a generous supply of glycine. Fibrosis, free radical damage, inflammation, cell death from ATP depletion or calcium overload, mitochondrial damage, diabetes, etc., can be prevented or alleviated by glycine …
Since persistent lipolysis and insulin resistance, along with a generalized inflammatory state, are involved in a great variety of diseases, especially in the degenerative diseases, it’s reasonable to consider using glycine/gelatin for almost any chronic problem.”
Red meat, on the other hand, contains far higher levels of the antimetabolic amino acids cysteine and tryptophan, which you want less of if you struggle with degenerative and/or inflammatory conditions.
Glycine for Bleeding, Stroke and Muscle Spasms
In his article,23 Peat also argues that a wide variety of bleeding conditions can be successfully treated with glycine, and hence a collagen- or gelatin-rich diet. These include everything from nosebleeds and excessive menstrual bleeding, to bleeding ulcers, hemorrhoids and even stroke.
According to Peat, glycine, taken shortly after a stroke, limits the damage and accelerates recovery. Glycine may also be protective in epilepsy, by stabilizing nerves and raising the amount of stimulation required to activate nerves. Glycine also has antispastic effects that can help alleviate muscle spasms associated with multiple sclerosis (MS).
Conditions involving excess prolactin, serotonin and/or cortisol, such as autism, postpartum and premenstrual problems, Cushing’s disease, diabetes and impotence, may also benefit.
“In some of the older studies, therapeutic results improved when the daily gelatin was increased,” he notes. “Since 30 grams of glycine was commonly used for treating muscular dystrophy and myasthenia gravis, a daily intake of 100 grams of gelatin wouldn’t seem unreasonable, and some people find that quantities in that range help to decrease fatigue …
For adults, a large part of that could be in the form of gelatin. If a person eats a large serving of meat, it’s probably helpful to have 5 or 10 grams of gelatin at approximately the same time, so that the amino acids enter the blood stream in balance.”
Make Sure You Are Getting Some Collagen or Glycine
Importantly, while glycine, proline, hydroxyproline and alanine all have anti-inflammatory and other healing properties, the primary amino acids in red meat tend to induce and/or promote inflammation (listed in red in the chart above).
For that reason, I cut my egg and meat intake by 50% and replaced the protein with gelatin and collagen instead. Overall, I aim to have about one-third of my protein as collagen or gelatin.
As a general suggestion, a good maintenance dose is about 20 grams of collagen per day. If you’re trying to address a soft tissue injury, you may want to increase that to 40 grams a day. You can also help prevent the breakdown of collagen by eating antioxidant-rich foods and avoiding cigarette smoke, pollution,24 excessive alcohol consumption and sugary foods.25 Vitamin C-rich foods also aid in collagen production.
Collagen Types
While 29 different types of collagen have been scientifically identified, most supplements will contain one or more of just three of these, which are known simply as:26,27,28
- Type 1 — Collagen found in skin/hide, tendon, scales and bones of cows, pigs, chicken and fish
- Type 2 — Formed in cartilage and typically derived from poultry
- Type 3 — Fibrous protein found in bone, tendon, cartilage and connective tissues of cows, pigs, chicken and fish
Types 1, 2 and 3 comprise 90% of the collagen found in your body.29 Collagen supplements typically come in one of two forms: unhydrolyzed (undenatured) or hydrolyzed (denatured) collagen. In their natural, hydrolyzed state, collagen molecules are poorly absorbed due to their large size.
Hydrolyzation refers to a processing technique that breaks the molecules down into smaller fragments, thereby enhancing intestinal absorption. For this reason, most collagen products are hydrolyzed.
Collagen Vs. Gelatin
As for the difference between collagen and gelatin: Collagen is the raw material and gelatin is what you get when you cook the collagen.30 While collagen and gelatin have the same basic amino acid composition, gelatin is more digestible and easier to absorb, which is important if your digestion is in any way compromised.
Collagen is made from animal bones, skins, tendons and other connective tissues. The collagen is extracted through an acid or alkali treatment followed by purification and does not involve heat. Since the molecular structure is larger, collagen does not dissolve in water.
When collagen is heated, the molecular bonds break down, giving you gelatin hydrolysate or hydrolyzed gelatin (other terms to describe gelatin include collagen hydrolysate or collagen peptides). Since the peptide chains are shorter, gelatin can be dissolved in water, where it forms a thick gel.
In terms of health benefits, these differences are likely minimal, because when collagen is ingested, it gets broken down in your gastrointestinal tract into shorter peptides that are the same as gelatin.
Since only free amino acids can enter your bloodstream, collagen and gelatin have essentially identical systemic effects, as their basic composition is the same. That said, gelatin may be preferable if you have ulcers or other GI problems.
Collagen Sources — The Good, the Bad and the Ugly
The ideal source of collagen/gelatin is homemade broth made from boiled organic chicken feet or beef bones. (Gelatin is the thickened layer that forms on top.) This also tends to be the most cost effective.
As far as supplements go, my preference is powdered gelatin, followed by collagen products made from beef bone broth rather than hide. When made from cattle hide, even organic certification becomes questionable, because hides, organic or not, are scraps from the leather tannery industry that have undergone intense processing with harsh chemicals.
Whether you choose gelatin or collagen, make sure it’s certified “100% Organic” by the U.S. Department of Agriculture (USDA)31 or, better yet, certified grass fed by the American Grassfed Association (AGA), which has the most rigorous standards.
Nonorganic collagen supplements are best avoided, as most are made from animal parts derived from animals raised in concentrated animal feeding operations (CAFOs), and may contain unwanted contaminants, including heavy metals,32 chemicals and drugs,33,34 including antibiotics.
Also, do not use JELL-O brand35 “gelatin” snacks, as ready-to-eat JELL-O cups contain no gelatin whatsoever. Instead, they’re using carrageenan, which can induce inflammation and contribute to a wide variety of chronic diseases.36 It can also cause digestive side effects.37
JELL-O powder38 does contain gelatin, as it contains food coloring and preservatives with questionable safety. What you want is a pure gelatin powder without sugar and other additives.
If you cannot afford a high-quality collagen or gelatin supplement, you could consider taking pure glycine instead. It’s available in powder form and tends to be very affordable and is easy to take, as it has a mildly sweet flavor. That said, alanine and proline have many of the same benefits as glycine, including protection against cell damage, so using gelatin rather than pure glycine is preferable.39
- 1 Skin Pharmacology and Physiology 2014; 27: 47-55 (PDF)
- 2 Journal of Medical Nutrition & Nutraceuticals 2015; 4(1): 47-53
- 3 J Drugs Dermatol. 2019 Jan 1;18(1):9-16
- 4 Bone 2010 Mar;46(3):827-3
- 5 PLoS One 2014 Jun 13;9(6):e99920
- 6 J Agric Food Chem. 2010 Jan 27;58(2):835-41
- 7 J Pharmacol Sci 2012; 118: 145 – 148 (PDF)
- 8 Curr Med Res Opin. 2008 May;24(5):1485-96
- 9 Curr Med Res Opin. 2006 November; 22(11):2221-32
- 10 Am J Physiol 1982 February;242(2):G85-8
- 11, 24 USA Today August 26, 2023
- 12 J Med Food. 2016 Sep;19(9):836-43
- 13, 14 J Med Food. 2010 Apr;13(2):399-405
- 15, 25 ABC15 August 21, 2023
- 16 Molecular Cell Biology, 4th Edition, Section 22.3. 2000
- 17 Amino Acids 2018 Jan;50(1):29-38
- 18 Science April 7, 2022; 376(6589): 188-192
- 19 National Natural Science Foundation of China, Chinese Scholars and Cooperators Achieved Progress in Bioprocessing-inspired Fabrication
- 20 Phys.org April 8, 2022
- 21 Umzu. Who Is Ray Peat?
- 22, 23, 39 RayPeat.com Gelatin Stress and Longevity
- 26 Nutraingredients.com March 19, 2015
- 27 Charlotte’s Book, Collagen Supplements
- 28 Amino-collagen.com, Types of Collagen
- 29 Woundresearch.com, A Review of Collagen and Collagen-Based Wound Dressings
- 30 Paleo Leap, Collagen Versus Gelatin
- 31 USDA.gov, USDA Organic
- 32 Rodale’s Organic Life May 19, 2017
- 33 Consumer Wellness Center October 5, 2017
- 34 Bonebroth.news October 5, 2017
- 35 Amazon JELL-O
- 36 MedicineNet Carrageenan
- 37 NIH. Environ Health Perspect. 2001 Oct; 109(10): 983–994
- 38 Amazon JELL-O Powder
The Wide-Ranging Health Benefits of Taurine
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2023/07/28/health-benefits-of-taurine.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola Fact Checked July 28, 2023
STORY AT-A-GLANCE
- The amino acid taurine is found in animal foods such as seafood, grass-fed red meat, dairy products, pastured eggs, and poultry
- Of the amino acids, it’s the most abundant source of sulfur, and is required for many biological processes, including the healthy function of your immune system, nervous system, metabolism, and digestion
- Taurine is important for brain and heart health, muscle function, bile salt formation and antioxidant defenses. It also helps rebuild damaged collagen fibers and can help ease anxiety
- According to recent research, taurine may also play an important role in longevity and healthy aging. In mice, the median lifespan increased by 10% to 12%. Life expectancy at 28 months was raised by 18% to 25%
- Taurine improved strength, coordination and endurance, bone mass and bone quality, glucose homeostasis and glucose tolerance, age-related inflammation, immune function, gut health, memory, mitochondrial function and the function of all organs
The amino acid taurine is found in animal foods such as seafood, grass-fed red meat, dairy products, pastured eggs, and poultry. Of the amino acids, it’s the most abundant source of sulfur, and is required for a wide range of physiological processes, including the healthy function of your immune system, nervous system,1,2 metabolism and digestion.3
As noted in a 2021 scientific review titled “The Role of Taurine in Mitochondrial Health: More Than Just an Antioxidant”:4
“Taurine is a naturally occurring sulfur-containing amino acid that is found abundantly in excitatory tissues, such as the heart, brain, retina and skeletal muscles …
Accumulating studies have shown that taurine supplementation also protects against pathologies associated with mitochondrial defects, such as aging, mitochondrial diseases, metabolic syndrome, cancer, cardiovascular diseases, and neurological disorders.”
Mechanisms of Action
More specific mechanisms of action of taurine include but are not limited to:
| Stabilizing proteins5 |
| Enhancing the function of endogenous antioxidants, thereby supporting your body’s ability to defend against oxidative/reductive damage.6 It protects your antioxidant status in several ways, including by neutralizing hypochlorous acid,7 diminishing the generation of superoxide by the mitochondria,8 and by minimizing oxidative stress,9 including mitochondrial oxidative stress induced by toxins10 |
| Reducing insulin resistance, hyperglycemia, and glucose serum concentrations11,12 |
|
Lowering lipid peroxidation13,14 |
| Reducing inflammation and associated organ injury15,16 |
| Conjugating cholesterol into bile acids, thereby aiding digestion and absorption of fats17 |
| Enhancing electron transport chain activity by regulating mitochondrial protein synthesis, and protects mitochondria against excessive superoxide generation18 |
| Neuroprotection, by regulating intracellular calcium;19 protecting against age-related memory degradation |
| Protecting against ionizing radiation-induced cell damage20 |
| Regulating sodium and calcium homeostasis21 |
| Reducing plasma LDL and triglycerides, and lowering cholesterol in the liver, thereby retarding development of atherosclerosis22 |
| Regulating gene expression by up-regulating 87 known genes and down-regulating 206 known genes in the liver, many of which are involved in cell growth, division, differentiation and apoptosis23 |
| Antiepileptic activity24 |
| Easing anxiety by increasing glycine and GABA25 |
Importantly, taurine can also help raise your metabolic rate, thereby serving as a useful aid against obesity and overall health optimization. Being similar in structure to glycine, it may also have anti-estrogenic effects.26
Symptoms and Health Effects of Taurine Deficiency
The fact that taurine can only be found in the foods that globalists are now trying to eliminate from our food supply is one of many reasons to push back and refuse their fake lab-made alternatives. Without natural animal foods, taurine deficiency will likely skyrocket and health may suffer across the board. Common symptoms and effects of taurine deficiency include:27,28,29,30
| Fatigue and low energy, as taurine is involved in energy production |
| Muscle cramps, muscle weakness, muscle wasting/atrophy and poor exercise performance, as taurine is essential for muscle health and function |
| Increased oxidative stress and systemic inflammation, which contributes to and is a hallmark of most chronic diseases |
| Impaired immune function, as taurine is involved in immune cell function and the regulation of inflammation |
| Vision problems associated with retinal degeneration, as taurine is essential for development and maintenance of the cells in your retina |
| Cardiovascular problems such as high blood pressure, irregular heart rhythm and cardiovascular diseases, as taurine helps regulate blood pressure31 and heart function.32
Taurine deficiency is common among patients with congestive heart failure (CHF),33 and oral supplementation has been linked to improved cardiac performance,34 improved contractile function of the heart,35 reduced pathology in the left main artery wall,36 and lower CHF mortality37 |
| Digestive problems such as bloating, diarrhea and malabsorption, as taurine is involved in bile production (and hence digestion and absorption of fats) |
| Accelerated aging, as taurine is slowing DNA damage, slows degradation of telomeres, improves mitochondrial function and is involved in cellular senescence; low taurine has been linked to a wide variety of age-related diseases in humans38 |
| Obesity, insulin resistance and diabetes39,40,41 |
| Liver disease |
Of course, since taurine is one of the nutrients missing in plant-based diets, vegans may want to consider a high-quality taurine supplement. While your body can synthesize some taurine, it’s not going to be sufficient in the long run, especially as you get older and your body’s ability to synthesize it diminishes.
Taurine levels decrease by an estimated 80% over the course of the average lifetime,42 and this decline is in part related to a loss of endogenous synthesis capacity over time. Still, the amount of taurine you get from your diet also plays a role, and even young healthy vegans have approximately 20% lower taurine levels than their meat-eating counterparts.43

Download this Article Before it Disappears
Taurine Supplementation Improves Exercise Performance
If you’re a fitness buff like me, you’ll be pleased to know that taurine can also help improve your athletic performance and reduce muscle damage. As reported in a systematic review published in 2021:44
“From the selected literature, we observed that taurine supplementation (2 g three times daily) with exercise can decrease DNA damage. Furthermore, 1 g of acute taurine administration before or after exercise can decrease lactate levels.
However, acute administration of taurine (6 g) at a high dose before the start of exercise had no effect on reducing lactate level, but increased glycerol levels, suggesting that taurine could be an effective agent for prolonged activities, particularly at higher intensities …
Finally, we observed that a low dose of taurine (0.05 g) before performing strength enhancing exercises can decrease muscular fatigue and increase enzymatic antioxidants …
This review systematically reported the dose response of taurine in improving exercise performance. We found from the selected literature that endurance training requires a higher dose of taurine, ~1 g five times daily, to prevent muscle-related damage whereas strength exercise requires a lower dose of taurine (0.05 g) to increase enzymatic antioxidants and decrease muscular fatigue.”
It also helps rebuild damaged collagen fibers, which can help you recover and recuperate from sports injuries faster.45
Taurine Increases Longevity
More recent research, published in the June 2023 issue of the journal Science,46,47 also found that oral taurine supplementation significantly increased the healthy lifespan of a variety of animals. In mice, the median lifespan increased by 10% to 12%. Life expectancy at 28 months was raised by 18% to 25%. In the video at the top of the article, biohacker and author Siim Land reviews these findings.
As reported by Science Alert:48
“Scientists have discovered not only that animals age more quickly when they don’t have enough of the amino acid taurine in the body, but that oral taurine supplements can delay aging and increase a healthy lifespan …
‘For the last 25 years, scientists have been trying to find factors that not only let us live longer, but also increase health span, the time we remain healthy in our old age,’ says biologist Vijay Yadav from Columbia University, senior author on the study. ‘This study suggests that taurine could be an elixir of life within us that helps us live longer and healthier lives.’”
Taurine Protects Against Hallmarks of Aging
Animals given supplemental taurine didn’t just live longer, they were also healthier overall. In mice, taurine improved:
| Strength, coordination, and endurance | Bone mass and bone quality |
| Glucose homeostasis and glucose tolerance | Age-related inflammation |
| Immune function | Gut health |
| Memory | Function of all organs |
| Mitochondrial function and health |
Interestingly, according to the authors, taurine “cured” osteoporosis. It’s not often you see the word “cure” being used in medical literature. Taurine also “suppressed ovariectomy-induced body-weight gain in a rodent model of menopause.”
Treated mice also had less body fat (approximately 10% less at 1,000 milligrams of taurine per day) and higher energy levels. According to the authors, “Fat-pad weight divided by body weight percentage was dose-dependently reduced in taurine-treated mice.”
As shown in previous studies, taurine supplementation also improved several markers of aging, including:
| Senescence | Intercellular communication |
| Telomere length | Epigenetic changes |
| Genomic stability | Mitochondrial function |
| Stem cell populations | Nutrient sensing |
Taurine Effects in Monkeys
Similar effects were observed when feeding taurine to rhesus monkeys. Fifteen-year-old monkeys (equivalent to 45 to 50 years old in humans) were given 250 mg per kg of bodyweight (equivalent to the 1,000 mg/kg given to mice) once a day for six months.
Compared to controls, taurine-fed monkeys gained less weight and had lower rates of body fat. After six months, they also had higher bone density, confirmed by higher serum markers for bone formation (osteocalcin) and decreased resorption. They also had 19% lower fasting blood glucose, a 20% to 36% reduction in liver damage markers, and a significant reduction in indirect markers of ROS-induced molecular damage.
“Thus, taurine has beneficial effects on most tested health parameters (body weight, bone, glucose, liver, and immunophenotype) in nonhuman primates,” the authors concluded.49
Previous animal research50 on mice lacking the taurine transporter also suggests taurine is involved in a wide variety of biologically protective processes, as these mice ended up developing multiorgan dysfunction. This too supports the notion that taurine is a key player in longevity and healthy lifespan.
Taurine in the Treatment of Stroke
Stroke victims may also benefit from taurine. As explained in a 2013 paper titled “Neuroprotective Mechanisms of Taurine Against Ischemic Stroke”:51
“Ischemic stroke (cerebral ischemia) is due to a partial or complete reduction in blood flow to the brain … Insufficient oxygen and glucose supply in cerebral ischemia leads to unsustainable cellular homeostasis which initiates cell injury.
Cellular injury progresses as a result of excitotoxicity, ionic imbalance, oxidative and nitrosative stresses, endoplasmic reticulum (ER) stress and mitochondrial disturbances, ultimately resulting in programmed cell death …
Taurine is able to cross the blood-brain barrier and displays a plethora of functions in the central nervous system (CNS) … Although taurine is not definitively classified as a neurotransmitter it fulfills most of the necessary criteria …
It modulates neurotransmission by eliciting inhibitory neuronal transmission through GABAA receptors, glycine receptors and putative taurine receptors … The fundamental pathophysiological mechanisms involved in ischemic stroke are glutamate excitotoxicity, calcium imbalance and oxidative stress which individually or collectively results in cell death.
Therefore, taurine’s role as an inducer of inhibitory neurotransmission, an antioxidant, neuromodulator, regulator of calcium homeostasis and neuroprotector, potentially makes it an ideal therapeutic agent for ischemic stroke.”
Taurine for Neurodegenerative Diseases and Post-Jab Injuries
Taurine may also be an important aid in the treatment of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. The reason for this is because taurine deficiency is associated with endoplasmic reticulum stress,52 a major contributor to prion diseases. Taurine is also thought to be important for proper protein folding.53
Disturbingly, the SARS-CoV-2 spike protein — introduced by natural infection or the mRNA COVID jabs — can pass through the blood-brain-barrier and cause damage resulting in everything from brain fog and dementia to Creutzfeldt-Jakob disease (human mad-cow disease),54,55,56 so taurine may also be valuable in the treatment of COVID, long-COVID and/or post-jab injuries.
Taurine Is Important From Cradle to Grave
As noted by the authors of the 2023 Science study, taurine’s effects on established hallmarks of aging makes it a veritable fountain of youth:57
“Although we do not yet know the initial events that taurine elicits, we provide evidence for the suppressed taurinylation of mitochondrial tRNAs during aging in mitochondrial dysfunction, a prominent feature of aging …
We propose that a combination of taurine and taurine-derived biomolecules may delay aging by affecting various aging hallmarks in distinct cells and tissues …
[D]uring early life, taurine appears to be essential for homeostasis in several organ systems, and its deficiency during development may compromise these functions postnatally.
Consistent with this hypothesis, organisms have a three- to fourfold higher taurine concentration in embryonic tissues than in adult tissues; moreover, taurine deficiency during development leads to growth retardation, blindness, and osteoporosis, and its supplementation during gestation increased bone mass postnatally …
It is possible that developmental or postnatal changes in taurine metabolism might affect the rate of aging during late life, and adjusting this endogenous machinery might extend healthy life span.”
- 1 Healthline Taurine
- 2 Journal of Biomedical Science August 24, 2010; 17 Article No. S1
- 3 Medical News Today Taurine
- 4 Molecules August 2021; 26(16): 4913
- 5 Amino Acids 2018; 50(1): 125-140
- 6 Molecular Medicine Reports June 24, 2021, Article No. 605
- 7 Amino Acids January 2014; 46(1): 89-100
- 8 Amino Acids January 2014; 46(1): 47-56
- 9 Mol Cell Biochem May 2016; 416(1-2): 11-22
- 10 Can J Physiol Pharmacol February 2009; 87(2): 91-99
- 11 Experimental and Molecular Medicine November 30, 2012; 44(11): 665–673
- 12 Food Chemistry: Molecular Sciences July 30, 2022; 4: 100106
- 13 Adv Exp Med Biol 1998;442:163-168
- 14 International Journal of Gerontology September 2011; 5(3): 166-170
- 15 Nutrition Journal 2021; 20: 53
- 16 Cellular Immunology May 2022; 375: 104503
- 17 Biocrates, Taurine
- 18 Amino Acids June 2012;42(6):2223-2232
- 19 J Neurosci Res November 15, 2001; 66(4):612-619
- 20 Advances in Experimental Medicine and Biology Book Series, Taurine 11, Pages 443-450
- 21, 34 International Journal of Clinical Cardiology, DOI: 10.23937/2378-2951/1410246
- 22 Amino Acids 2014; 46: 73-80
- 23 J Med Food Spring 2006; 9(1): 33-41
- 24 Riv Neurol October-December 1975; 45(4): 391-398
- 25 Anxiety Medication, Taurine
- 26 Ray Peat, Gelatin, Stress, Longevity
- 27, 32 NAO Medical Symptoms of Taurine Deficiency
- 28, 39, 42, 43, 46, 49, 57 Science June 9; 380(6649) DOI: 10.1126/science abn9257
- 29, 40 Biomolecules & Therapeutics 2018; 26(3): 225-241
- 30, 41 Twitter Dr. Eashwarran Kohilathas April 7, 2023
- 31 European Journal of Pharmacology May 3, 1990; 180(1):119-127
- 33 Hearts 2020; 1(2): 86-98
- 35 American Heart Journal June 2002; 143(6): 1092-1100
- 36 Hypertension April 27, 2009; 53:1017–1022
- 37 Journal of Biomedical Science 2010; 17 Article No. S6
- 38 Science June 9, 2023; 380(6649)
- 44 Frontiers in Physiology 2021; 12: 700352
- 45 Tohoku J Exp Med March 2015; 235(3): 201-213
- 47 Science June 8, 2023; 380(6649): 1010-1011, Perspective/Commentary
- 48 Science Alert June 12, 2023
- 50 Nutrients 2017; 9(8): 795
- 51 Brain Sciences June 2013; 3(2): 877-907
- 52, 53 Adv Exp Med Biol 2015; 803: 481-487
- 54 Prion 2022; 16(1): 78-83
- 55 Cureus February 2023; 15(2): e34872
- 56 Twitter Dr. Eashwarran Kohilathas February 15, 2023
Glycine to Increase Longevity and Decrease Depression
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2023/04/20/glycine-increase-longevity-decrease-depression.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola Fact Checked April 20, 2023
STORY AT-A-GLANCE
- Glycine has been shown to extend lifespan in animal studies and mitigate chronic disease and disability, thereby increasing healthspan
- Glycine has anticancer effects, reduces insulin and alleviates neuroinflammation; it may also protect against depression and is essential for collagen synthesis
- To gain all of glycine’s healing potential, doses of 10 to 20 grams a day may be optimal
- You need at least 12 grams of glycine daily for optimal collagen turnover, plus another 3 grams per day to form glutathione
- Excess methionine from eating animal products without the connective tissues decreases longevity, but adding glycine will reduce the methionine/glycine ratio to counter the negative side effects of excess methionine
- Glycine has neurotransmitter qualities, improves depression and is also useful for improving sleep quality by helping relaxation at night by being very similar to the neurotransmitter GABA
Glycine, a nonessential amino acid, meaning your body can make it, but most of us as we age are simply unable to make enough of it, especially if our dietary intake is low because we are not eating enough connective tissue and collagen in our diet.
New research is emerging showing glycine is a powerful longevity enhancer, one that’s not only inexpensive but also has a pleasant, slightly sweet flavor. In fact, glycine is sometimes used as a sugar substitute, and I personally take 1 teaspoon with each of my two meals and before bedtime for its health-enhancing qualities.
Research shows glycine extends lifespan in worms, mice and rats while improving health in models of age-related disease.1 If there were any doubt about its importance, consider that collagen — the most abundant protein in your body2 — is made mostly of glycine. It’s also a precursor to glutathione, a powerful antioxidant that declines with age.
As noted by Siim Land, author of “Metabolic Autophagy,” in the video above,3 however, there are two glycine benefits that appear key to its actions as a veritable fountain of youth — inducing autophagy and mimicking the longevity benefits of methionine restriction.4
How Glycine May Influence Aging
Glycine is a receptor for the enzyme glycine N-methyltransferase (GNMT), which plays a role in methionine clearance, according to a review published in Ageing Research Reviews.5 Glycine is the acceptor for GNMT, an enzyme responsible for methionine clearance. GNMT converts glycine to sarcosine, “an autophagy-inducing metabolite.”6
Further, in mice deficient in GNMT, levels of free methionine may be seven-fold higher, while S-adenosyl-L-methionine may increase by 35-fold.7 This matters, as methionine is involved in cancer cell growth and metabolism, while methionine restriction inhibits cancer cell growth.8
Methionine restriction has been shown to improve longevity, extending lifespan in mice while lowering levels of insulin, glucose and insulin-like growth factor 1. Limiting methionine also yields a host of additional antiaging benefits in ice, like reducing liver damage after exposure to dangerous amounts of acetaminophen and reducing overall frailty.9
“Since a low level of methionine signifies a low nutrient state, methionine restriction is thought to act as a caloric restriction mimetic,” the Ageing Research Reviews report explains.10 Glycine, in turn, the researchers noted, “may prolong life by serving as a methionine restriction mimetic.”11 It does this because it lowers the methionine/glycine ratio which may be more important than the absolute level of methionine consumption.
You Need Glycine for Collagen Synthesis
Indeed, in a study on mice using data from the National Institute on Aging’s Interventions Testing Program, a team of scientists revealed that feeding a diet with 8% glycine increased lifespan significantly, by 4% to 6%, in males and females, while offering additional benefits like reduced risk of dying from lung cancer.12
Some animal studies have shown up to a 28.4% median increase in lifespan when eating a diet containing 8% or 12% glycine.13 There are also direct antiaging effects via collagen synthesis. Land explains:14
“Glycine also has a very important role in antiaging directly by helping to reduce wrinkles, and collagen synthesis. Glycine makes up every third amino acid in collagen, which is why there is such a large requirement of glycine for optimal collagen turnover.
The less collagen or glycine you consume, the slower your collagen turnover is. Slow collagen turnover increases the damage that occurs to your collagen, such as glycation and oxidation, and reduces collagen deposition into tissues.
Collagen is the most abundant protein in the human body, making up approximately 30% of all your protein by mass. It makes up your hair, teeth, skin, nails, organs, arteries, cartilage, bones, tendons and ligaments. Collagen is literally the glue that holds you together. So making sure that you preserve your collagen is very important for slowing down aging, especially when it comes to wrinkles.
Starting at the age of 20 you lose just under 10% of your skin’s collagen content every decade. So, by the time you’re 75 years old you would have lost 50% of your skin’s collagen content … up until very recently it was thought that the collagen turnover was very slow and it only happened over the course of many years — over the entire lifespan.
However, recently it was shown that college turnover happens every day and is part of your daily protein turnover.”

Download this Article Before it Disappears
Glycine Protects Against Age-Related Disease
Also significant is glycine’s potential to mitigate chronic disease and disability, thereby increasing healthspan throughout your body. Glycine receptors exist in the central nervous system, for instance, which means glycine acts as a neurotransmitter.15 The Ageing Research Reviews study compiled multiple examples of glycine’s ability to fight age-related disease in animals, including:16
| Suppressed tumor growth in mice with melanoma17 | Decreased fasting glucose, insulin, triglyceride and insulin-like growth factor 1 in male rats18 |
| Preserved muscle mass and reduced inflammatory markers in mice with cancer cachexia19 | Improved endothelial function in older rats20 |
| Even intermittently restricting methionine leads to benefits like improved glucose homeostasis, reduced obesity and protection against fatty liver | Reduced weight gain and improved bone mineral density in a mouse model designed to mimic postmenopausal bone loss21 |
| Protected against cardiac hypertrophy22 | Alleviated neuroinflammation and protected against cognitive deficits in mice with neurodegeneration23 |
Human trials confirm what the animal models suggest — that glycine is protective against a range of chronic diseases. In a study of 60 people with metabolic syndrome, 15 grams of glycine a day for three months had reduced oxidative stress and improved systolic blood pressure.24
In older adults, limited availability of glycine and cysteine may lead to decreased synthesis of glutathione — composed of the three amino acids cysteine, glycine and glutamic acid — such that glutathione deficiency is widespread in this population.25 The lack of glutathione, perhaps driven by limited glycine, in older adults may be a key element driving the oxidative stress and mitochondrial dysfunction that lead to age-related degeneration. Land notes:26
“The benefits of glycine generally have to do with improving the blood sugar levels, fasting insulin levels, triglycerides, even lowering the demand for sleep, improving brain function and health, helping with just overall aspects of vitality.
… a lot of the longevity benefits come from the methane restriction and the autophagy stimulation that pretty much helps to clean out the cells from the dysfunctional components as well as boosting glutathione levels, which just enables the body to function with less inflammation and oxidative stress, which is very crucial for aging and it also pretty much buffers against the methionine toxicity.”
Glycine With NAC Supports Mitochondrial Health
Researchers at Baylor College of Medicine also looked into supplementation with a combination of glycine and N-acetylcysteine (NAC), two glutathione precursors known as GlyNAC when taken together.
They had previously shown that young mice deficient in glutathione had mitochondrial dysfunction, and supplementing with GlyNAC in older mice not only improved glutathione deficiency but also mitochondrial impairment, oxidative stress and insulin resistance.27
Additional previous research they conducted in HIV patients28 found GlyNAC supplementation improved “deficits associated with premature aging” in this population.29 This included improvements to oxidative stress, mitochondrial dysfunction, inflammation, endothelial dysfunction, insulin resistance, genotoxicity, strength and cognition.30
A subsequent pilot trial in older humans found similar results, with GlyNAC supplementation for 24 weeks correcting glutathione deficiency and improving multiple measures of health, including:31
| Mitochondrial dysfunction | Oxidative stress | Inflammation |
| Endothelial dysfunction | Insulin resistance | Genomic damage |
| Cognition | Strength | Gait speed |
| Exercise capacity | Body fat levels | Waist circumference |
Further, GlyNAC supplementation improved four of nine hallmarks of aging associated with most age-related disorders — mitochondrial dysfunction, inflammation, insulin resistance and genomic damage.32 Glycine, the team noted, is an important methyl-group donor. “Methyl groups are abundant in DNA and are important components of multiple cellular reactions. Glycine is also important for normal brain function.”33
In addition to supporting brain function,34 supplemental glycine may be useful for the “prevention and control of atherosclerosis, heart failure, angiogenesis associated with cancer or retinal disorders and a range of inflammation-driven syndromes, including metabolic syndrome.”35
Glycine’s Link to Depression
As a major neurotransmitter,36 glycine’s role in brain health is receiving increasing attention. The results of a 15-year study conducted by University of Florida researchers also suggest it may be involved in depression. The finding relates to a receptor called GPR158. When suppressed in mice, stress-induced depression is less likely.
When they determined the structure of GPR158, they realized it’s an amino acid receptor — for glycine. “We were barking up the completely wrong tree before we saw the structure,” study author Kirill Martemyanov told Medical News Today. “We said, ‘Wow, that’s an amino acid receptor. There are only 20, so we screened them right away and only one fit perfectly … it was glycine.”37
After learning that GPR158 binds to glycine and acts as a metabotropic glycine receptor, they named it mGlyR.38 The team explained in the journal Science:39
“Glycine signals through mGlyR to inhibit production of the second messenger adenosine 3′,5′-monophosphate. We further show that glycine, but not taurine, acts through mGlyR to regulate neuronal excitability in cortical neurons. These results identify a major neuromodulatory system involved in mediating metabotropic effects of glycine, with implications for understanding cognition and affective states.”
Glycine is also useful for improving sleep quality.40 “It can help to relax at night by being very similar to GABA,” Land says. “… It’s beneficial for … reducing the time it takes to fall asleep. People who ingested 3 grams of glycine within one hour before bedtime saw an improvement in subjective sleep quality, fell asleep faster and were less sleepy during the day.”41
How Much Glycine Is Enough?
To gain all of glycine’s healing potential, doses of 10, 15, or 20 grams a day may be necessary. Land suggests you need at least 12 grams of glycine daily for optimal collagen turnover, plus another 3 grams per day to form glutathione and other compounds:42
“Your body only makes 3 grams of glycine per day, and if you only consume around 2 to 3 grams of glycine from foods then it means that almost all of us are in a 10-gram glycine deficit every day,” he says.
“… I think most people would benefit for at least 5 to 10 grams of glycine a day, which is, uh kind of a moderate amount … if you are eating a lot of muscle meat … or you’re just interested in getting more of the benefits of glycine then you can take even up to 20 grams a day.”
In addition to supplements, collagen is an outstanding source of glycine. My personal preference is to use a less denatured (unhydrolyzed) organic collagen supplement, as it has a more balanced amino acid profile or, better yet, simply boost your collagen intake by making homemade bone broth using bones and connective tissue from grass fed, organically raised animals.
– Sources and References
- 1 Ageing Research Reviews March 31, 2023, Highlights
- 2 Cold Spring Harb Perspect Biol. 2011 Jan; 3(1): a004978
- 3 YouTube, Siim Land, Glycine Longevity Benefits Are Amazing – New Study Confirms April 5, 2023
- 4, 5, 6, 7, 8, 9, 10, 11 Ageing Research Reviews March 31, 2023
- 12 Aging Cell January 23, 2019
- 13 Ageing Research Reviews March 31, 2023, Table 1
- 14 YouTube, Siim Land, Glycine Longevity Benefits Are Amazing – New Study Confirms April 5, 2023, 5:49, 7:31
- 15 Ageing Research Reviews March 31, 2023, Intro
- 16 Ageing Research Reviews March 31, 2023, Table 2
- 17 Carcinogenesis, Volume 20, Issue 5, May 1999, Pages 793–798
- 18 The FASEB Journal April 1, 2011
- 19 Clinical Nutrition June 2014, Volume 33, Issue 3, Pages 448-458
- 20 Canadian Journal of Physiology and Pharmacology June 2015, Volume 93, Number 6
- 21 Amino Acids volume 48, pages 791–800 (2016)
- 22 Biochemical Pharmacology January 1, 2017
- 23 Journal of Neuroinflammation October 15, 2020
- 24 Canadian Journal of Physiology and Pharmacology June 17, 2013
- 25 Innov Aging. 2019 Nov; 3(Suppl 1): S416
- 26 YouTube, Siim Land, Glycine Longevity Benefits Are Amazing – New Study Confirms April 5, 2023, 5:49, 7:00
- 27 Clinical and Translational Medicine March 27, 2021, Abstract, Background
- 28, 30 Biomedicines September 30, 2020
- 29 Baylor College of Medicine March 29, 2021
- 31 Clinical and Translational Medicine March 27, 2021
- 32 Clinical and Translational Medicine March 27, 2021, Section 4.12
- 33, 34 Clinical and Translational Medicine March 27, 2021, Section 4.13
- 35 Medical Hypotheses January 15, 2019
- 36, 38, 39 Science March 30, 2023, Abstract
- 37 Medical News Today April 4, 2023
- 40 Neuropsychopharmacology. 2015 May; 40(6): 1405–1416
- 41 YouTube, Siim Land, Glycine Longevity Benefits Are Amazing – New Study Confirms April 5, 2023, 5:49, 8:50, 11:56
- 42 YouTube, Siim Land, Glycine Longevity Benefits Are Amazing – New Study Confirms April 5, 2023, 5:49, 8:50, 13:46
How to Stay Fit for Life
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2023/01/08/how-to-be-healthy-and-strong-in-old-age.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola Fact Checked January 08, 2023
STORY AT-A-GLANCE
- It is rarely too late to start resistance training; you can build muscle mass well after age 60. In 2022, I set a new personal record in the leg press for 600 pounds, far better than the 400-pound deadlift I did the year before
- Sarcopenia — age-related muscle loss — threatens a healthy lifespan. Skeletal muscle not only manages physical activity, but also plays a major role in metabolism, circulation and cognition
- Skeletal muscle acts as endocrine organ secreting myokines and transcription factors into the bloodstream, thereby regulating the function of other organs. It also has immune regulatory properties
- The loss of muscle mass is thought to be a primary driver of insulin resistance in older adults. Declining muscle strength and reduced physical activity also contribute to metabolic dysfunction
- In order to gain muscle mass though one needs a critical to eat enough protein. A minimum is about 1.2 grams per kilogram of body weight (0.54 grams/pound) per day. Athletes and the elderly could probably benefit from going up from that level to about 1.6 grams/kilogram (0.71 grams/pound)
This may be one of the most important articles I’ve ever written with respect to helping you understand how important resistance exercise is to not only slowing down the aging process but improving your overall metabolic health. We know this is important because studies have shown that over 75% of those over 65 do not exercise enough to stay healthy.
But, the key is to understand the type of exercise that will give you the greatest benefit for the time invested. If we had to engage in hard physical labor for our work we wouldn’t need to exercise. Formal exercise is only needed because most of us have long ago stopped engaging in manual labor. Modern society has allowed us to obtain food and shelter with relatively little effort. So, we need exercise to compensate for this if we hope to optimize our physical health.
I want to share my experience with you so you don’t make the same mistakes I did 50 year ago. To me the evidence is crystal clear: As you age you need to engage in some type of resistance training to compensate for the degeneration that typically accompanies aging and decreased physical activity. You can do cardio if you have the time, but not at the expense of building lean muscle mass. This is largely because aging accelerates muscle loss.
Understanding the Hazards of Sarcopenia
The medical term for age-related muscle loss is sarcopenia.1 Sarcopenia is derived from two Greek words: sarx (flesh) and penia (poverty).2 So, as you get older you will invariably start losing muscle mass, and if you don’t engage in resistance exercises, you will likely suffer metabolic diseases as well.
An estimated 25% of 60-year-olds have sarcopenia, and nearly two-thirds of those 80 years and older have lost serious amounts of muscle mass, which threatens a healthy lifespan and cuts down on your independence and quality of life.3,4 Sarcopenia leads to many functional limitations, including difficulties in walking, climbing stairs and carrying objects.5 The penalties of this functional decline include falls, disability, institutionalization6 and even death.7
One of the reasons I’m committed to lifelong exercise is because both of my parents died from frailty, and I’m determined to avoid sarcopenia, which took them prematurely. This was a powerful motivation for me to deeply study frailty so I could avoid their fate.
With society aging worldwide, the prevalence of sarcopenia increases the urgent need to establish prevention and intervention strategies. The U.S. Centers for Disease Control and Prevention now recognizes sarcopenia as an independently reportable medical condition.8
Skeletal muscles not only function to generate force and movement, but also play a major role in your metabolism, circulation and cognition, as seen in the following figure. Skeletal muscles also serve an important endocrine function. They secrete special cytokines (i.e., myokines) and transcription factors into the bloodstream, thereby regulating the function of other organs. It’s a metabolically active tissue with an important role in the maintenance of metabolic homeostasis.

Sarcopenia Is a Major Driver of Insulin Resistance and Disease
Skeletal muscle is the most abundant tissue in your body, comprising 40%9 to 55%10 of your body mass, and is the primary sink of insulin-mediated glucose disposal. Muscle is also the major site for insulin-stimulated glucose uptake, as well as the main energy consumer of fat.11 After meals, about 80% of glucose is deposited in your skeletal muscle.12,13
The loss of muscle mass with advancing age is thought to be a primary driver of insulin resistance in older adults.14 Again, this is because muscle is the major tissue where insulin causes glucose to be absorbed.
But it doesn’t end there. Remember, muscle makes up nearly half of your body’s tissues, so once your body runs out of sugar in the form of glycogen, it uses fat, especially if you are metabolically flexible. So, muscle is also the main energy user of fat in your body.15 The declining muscle strength and progressive mobility impairment with age also tends to reduce daily physical activity, which also contributes to metabolic dysfunction.16,17
The loss of resilience as a result of sarcopenia is underappreciated as a major factor in the ability to recover from life’s inevitable challenges. It is clear that elderly with low muscle mass experience delayed recovery,18,19 and have higher rates of complications and infections following surgery,20 greater drug toxicity21 and higher disease-specific and all-cause mortality.22
Sarcopenia also predicts both the risk for community-acquired pneumonia in the elderly,23 as well as 90-day mortality in patients suffering from aspiration pneumonia.24
Muscle Play a Role in Your Immune Function Too
Muscle is increasingly recognized as an organ with immune regulatory properties. As such, skeletal muscle cells modulate immune function by signaling through different soluble factors, cell surface molecules or cell-to-cell interactions.25 It is also speculated that sarcopenia contributes to immunosenescence — the gradual deterioration of your immune system — which is a leading cause of death in the elderly.26
Additionally, recent reviews found strong evidence that frailty due to sarcopenia27 is a risk factor for adverse outcomes, such as longer hospital stay, functional decline at discharge and both in-hospital and medium, lower quality of life,28 and long-term mortality.29
My Strategy and Recommendation to Combat Sarcopenia
So, what can we do about this progressive decline in muscle loss that sets you up for frailty and metabolic catastrophe? Why, exercise, of course. But here’s a little-known fact: Despite the well-known benefits of resistance training, less than 10% of those under the age of 75 in the United States participate in muscle-strengthening activities.
I believe one of the main reasons for this low rate of participation is that over half of those who do exercise engage in conventional resistance training end up getting injured. Another reason is that conventional strength training is considerably less effective in healthy older adults than in young adults.
This blunted anabolic response to exercise training in older individuals30 is likely a result of the age‐related decline in muscle fiber perfusion.31
This has been known for a long time. Seventeenth century physician Dr. Thomas Sydenham, who is known as the “English Hippocrates,” recognized nearly 400 years ago that vascular health and aging are interdependent and inversely related.32 His famous quote is: “A man is as old as his arteries.”
Microcirculation is the term used to describe blood flow through the capillaries. The main function of the microcirculation is the delivery of oxygen and nutrients to tissues while removing CO2, metabolic debris and toxins. Researchers believe this is related to a decrease in muscle fiber microcirculation of Type II muscle fibers and their associated stem cells.
Studies have shown that Type II muscle fiber-associated stem cells are located at a greater distance to their nearest capillary in older compared with younger men.33 There are a large number of circulating growth factors that are regulators of stem cell function34 and the delivery of these signals to activate your muscle stem cells and promote muscle growth relies on how close they are to the capillaries.
Once your microcirculation becomes compromised with age, Type II muscle fibers and their associated stem cells will be unable to receive enough nutrients and oxygen. Thankfully, there is a solution to this dilemma, and it is called blood flow restriction (BFR) or KAATSU in Japan, which is where it originated.
How I Radically Changed My Exercise and Lean Muscle
Now, I am no stranger to exercise. I have been exercising since 1968, which is 54 years. The problem is that the first 43 years were exclusively cardio, and in my case long distance running. I like to compete, so I got relatively decent and was eventually able to run a 2:50 marathon, which was good enough back then to get me on the post-graduate University of Chicago Track Club.
Unfortunately, I didn’t realize that while cardiovascular exercise can lower your risk of heart disease, it is a highly catabolic activity and will actually lower your ability to build muscle. Below is a picture of me taken during my peak running condition. As you can see by the arrow, I had a “gigantic,” 10.5-inch arm circumference.

Contrast that to the picture below, taken December 8, 2020, where my arm circumference is 15 inches. I had just finished doing a PR video for the deadlift at 370 pounds. The video (below) was posted to Instagram on the same date.
The blue plates are 45 pounds each; the black ones are the same width as the blue ones, but are plastic and are 25 pounds; and the bar is 50 pounds. My team wanted me to do a story on how I did this to, hopefully, inspire you to similar, if not better, levels of strength.
I’m very proud to say that I’ve come even further since then and, now, even approaching 70, I’ve been able to regularly set personal records with some of the lifts that I’m doing, and have deadlifted four plates, which is 405 pounds.

Watch Me Leg Press 600 Pounds
Without doubt, building muscle is one of the most important strategies to improve and safeguard your health, especially as you age. You need protein reserves to survive serious disease, and most of your protein is stored in muscle. If you have very little muscle, you’re going to pass away prematurely because you have no amino acid reserves.
As mentioned, your muscle is also a primary regulator of your metabolism. It’s a primary site for glucose disposal because of the GLUT4 insulin receptors embedded in the muscle cell membranes. These receptors lower your glucose levels after a meal and decrease your risk for diabetes. It also interfaces with your immune system and helps optimize it.
If you take away one tip from this article, let it be this — it is rarely too late to start resistance training. You can build muscle mass after 60, which is about when I started and, earlier in 2022, as you can see in the video above, I set a new personal record in the leg press for 600 pounds, a significant improvement over the 400-pound deadlift I did in 2021.
How did I do it? That’s the focus of this article, and the great news is that virtually anyone can use this strategy, even if you’re older, like me, or already a bit frail, or have previously experienced exercise injuries.
The BEST Strategy I Know of to Increase Muscle Size
There are loads of ways to increase your muscle mass but they mostly involve moving, pushing or pulling heavy weights or resistance bands. The problem with this strategy is that if you are not in good shape, and especially if you are elderly, there is a very high likelihood that you will get injured. In most cases, it is not if you will get injured but when.
The answer to this problem is an exercise strategy known as BFR or KAATSU. As the name implies, BFR involves modifying the arterial inflow and venous outflow while you’re working the muscle by placing an inflatable band around the extremity.35 It is not like a tourniquet that stops all your blood flow, which is dangerous.
BFR was developed by Dr. Yoshiaki Sato of Japan about 50 years ago. In Japan, where it’s widely popular, it’s known as KAATSU, meaning “training with added pressure.”36 However since Sato does not speak or write English, the first article published about it in the U.S. was about 25 years ago.37
Conventional resistance training typically uses resistance at 70% to 85% of your one-rep max, i.e., the maximum amount of weight you can lift only one time. Since this weight is relatively heavy and close to your limit, injuries are almost guaranteed.
BFR is different, as it is a low-intensity resistance training, using weights that are just 20% to 35% of your one-rep max. With weights this light, your risk of injury is largely eliminated. In many elderly and frail individuals, weights of just 1 or 2 pounds, or no weight other than your body, are all that is needed to achieve the benefits.
How BFR Works
BFR’s ability to achieve such remarkable physiological benefits is directly related to slowing venous blood flow from the muscle group being engaged and creating a relatively hypoxic environment or low oxygen pressures in the exercising muscle.

Venous flow moderation is optimally achieved by wrapping the extremity being exercised with an inflatable cuff or band.
The band needs to be tight enough to slow venous return to the heart, allowing venous blood to “pool” in the region of the limb that is being exercised, while loose enough to allow arterial blood to flow through.
With very light exercise, and in about 15 to 20 minutes, you get an exhaustive workout that sends a signal to your brain that says, “Hey, I’ve done something really hard here — you better help me recover and adapt to it.”
Your brain then sends out hormonal responses that cause your muscles and blood vessels to grow. Most would think that such light weights would be insufficient to provide any muscle strength improvements, but studies show a 36% to 40% increase in muscle strength after only 12 weeks, depending on your load and health.38
BFR Mimics Heavy Weight Training Without Any of the Risks
BFR training is frequently misunderstood as simply a conventional resistance training program with the addition of resistance bands. Nothing could be further from the truth.
Because the exercise is done with such low weights, there’s far less muscle fiber trauma and damage, especially relative to conventional strength training. This means you are able to recover much quicker, so you don’t have to dig yourself out of a hole the next few days. In most cases, you can exercise different body parts nearly every day and rapidly attain the metabolic and physical benefits.
To understand the mechanism of BFR you need to know that you have two basic types of muscle fibers. First you have slow-twitch oxidative (Type I) fibers designed for low-intensity long-lasting contractions. Secondly, you have fast-twitch glycolytic (Type II) fibers designed for high-intensity short-duration contractions. When you lose muscle, it typically occurs as a reduction in Type II muscle fibers.

If you are going to increase muscle mass and strength in anyone, but especially the elderly, it is important to activate Type II muscle fibers during training, since these fibers have been shown to be far more responsive to growing muscle than Type I fibers39 and are generally much larger.
Weight training done at low weights will not activate Type II fibers — unless it’s done with BFR. Type I fibers are relatively more sensitive than type II fibers to hypoxia (low blood oxygen), as they have a greater oxygen consumption at rest compared to Type II fibers.40 BFR training takes advantage of this difference. Producing a relatively hypoxic environment in your muscles41 causes premature fatigue of the Type l fibers, thus forcing your body to rely on Type II fibers to continue the exercise.
At the same time, you’re also activating their associated muscle stem cells. This is likely one of the main mechanisms by which BFR can trigger muscle growth and prevent or prevent or treat sarcopenia. Simply moving light weights with high repetition without BFR will not engage Type II fibers because there is plenty of oxygen for the Type I fibers to work. Hence, the fast-twitch Type II fibers just aren’t called into action.
How BFR Affects Your Muscles and Overall Health
When you exercise and activate your Type 2 fibers with BFR, your muscles will generate a waste product called lactic acid or lactate, which is responsible for much of the metabolic magic. Activating Type 2 fibers also lowers the pH of the muscles. This is not because of lactic acid, but more related to the release of extra protons released in generating energy.42
Both lactate and proton accumulation are potent stimulators of growth hormone and insulin-like growth factor (IGF-1), which in turn leads to muscle growth.43 IGF-1 is a hormone that helps manage growth hormone (GH) in the body. It’s typically made by your liver, which is the largest contributor to IGF-1 circulating in your blood. However, when your liver secretes IGF-1, it will not act on those tissues that have capabilities of producing the hormone themselves, such as skeletal muscle.44
Interestingly, it is not the circulating levels of IGF-1 in your blood that cause your muscles to grow but, rather, the IGF-1 produced by your muscles when engaged in exercises like BFR, as that’s the key determinant for switching on the anabolic muscle building pathways.
While high levels of IGF-1 in your blood will inhibit autophagy and decrease your longevity,45 this does not appear to be the case when you increase IGF-1 in your muscle using anaerobic exercises like BFR. This IGF-1 does not leak out into your blood to suppress autophagy.46
BFR will not only will add solid muscle mass, but also significantly increase your strength and endurance while reducing your body fat. For most people who are not competitive athletes, it’s really the only form of resistance training they need.
Competitive athletes also seem to benefit from BFR, but they would need to combine it with conventional strength training.47 In short, BFR works on a very simple principle: It tricks your body into believing that it’s moving far heavier weights than you’re actually using, and as a result generates compensatory metabolic responses, further detailed below.
Local and Systemic Effects of BFR
If you are elderly, what is really amazing is that your muscle growth with BFR is beyond what strength training with heavy weights can do. This is because you need good blood flow to your Type II muscle fiber stem cells, and as mentioned earlier, virtually everyone’s microcirculation decreases with age.
So, even if you send the signal to grow by doing conventional strength training, it won’t work as well if there isn’t enough capillary supply to your Type II fiber stem cells. BFR increases your microcirculation, your capillaries and venules and arterioles that are associated with them (see image below), largely because your muscles are working in a hypoxic (low oxygen) environment.
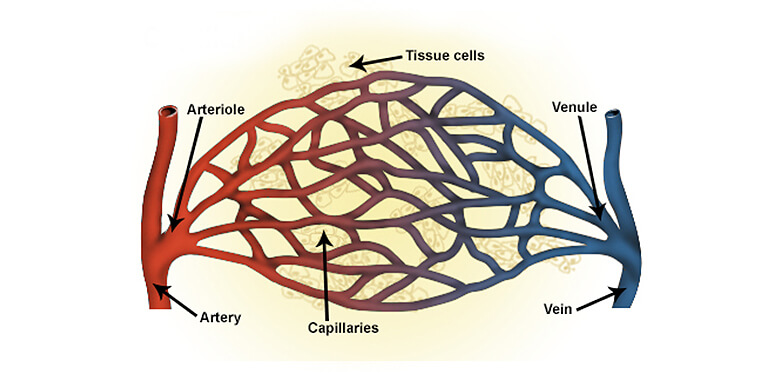 Microcirculation
MicrocirculationHow BFR Increases Your Microcirculation
This low oxygen tension causes the release of hypoxia-inducible factor-1 alpha (HIF-1 alpha),48 which in turn increases the hormone vascular endothelial growth factor (VEGF), which is one of the most powerful angiogenic signals in your body. It’s an extremely potent pro-blood vessel building cytokine or myokine. VEGF will help repair damage that has occurred to your blood vessels, improve elasticity and make them far more resilient to damage and accidents.
BFR has been shown to raise VEGF levels by 410% in young adults.49 Essentially it acts as “fertilizer” for growing new blood vessels and capillaries to your muscle stem cells. BFR training has been shown to increase muscle stem cells by 300% after eight days of training.50
Here is the KEY point: The VEGF released by BFR is systemic and carried in your blood to your entire body. It just doesn’t work on the limbs you are exercising. It increases blood vessel growth throughout your entire body, which seems to be the perfect antidote for Dr. Syndenham’s theory that a man is old as his arteries.
In short, BFR will help aging men have the arteries of boys. VEGF also increases microcirculation in your brain and heart. In Japan, BFR is frequently used for stroke and cardiac rehab precisely for this purpose.
BFR also increases the production of the important regulatory free radical, nitric oxide (NO), which further contributes to an increase in VEGF.51 NO is an important signaling molecule produced at high levels in muscle by neuronal nitric oxide synthase (nNOS). BFR, by way of increasing NO, has been found to stimulate muscle satellite stem cells and proliferation.52
There is a load of interest in NAD+, as it’s a primary fuel for longevity proteins, and it becomes depleted as you age. A combination of BFR along with 50 milligrams of niacinamide per day will radically increase NAMPT, which is the rate limiting enzyme for NAD+. Honoring your circadian rhythm is also an important part of the equation.
The Benefits of Osmotic Pressure

Metabolic stress, or the accumulation of metabolites during exercise, has been documented to be important for muscle growth.53,54,55 One of the most important metabolic stressors produced in BFR training is lactate, which I touched on earlier. The lactate released by the muscles is largely a result of Type II muscle fiber burning glucose into pyruvate and then converting pyruvate to lactate.
When you occlude the veins in BFR, you limit the venous blood flow return back to your heart, which allows the lactate to accumulate to high concentrations in your muscle,56 where it creates an osmotic pressure differential that requires the influx of water to normalize.
As you can see by the figure below, osmotic pressure is the pressure that must be applied to a solution to halt the flow of solvent molecules through a semipermeable membrane (osmosis). It is determined by how many molecules are in the solution. The more molecules, the higher the osmotic pressure.
This flow of water into the muscle57 contributes to intracellular swelling,58 which creates an acute and measurable increase in the size of the muscle that is typically quite visually obvious.
The mechanical pressures created by this cell swelling also trigger the activation of Type II muscle fiber stem cells, which causes muscle growth and further increases muscle protein synthesis and decreases protein breakdown in your muscles.59 So, the higher the lactate levels and swelling created with BFR training, the better the muscle-building results will be.
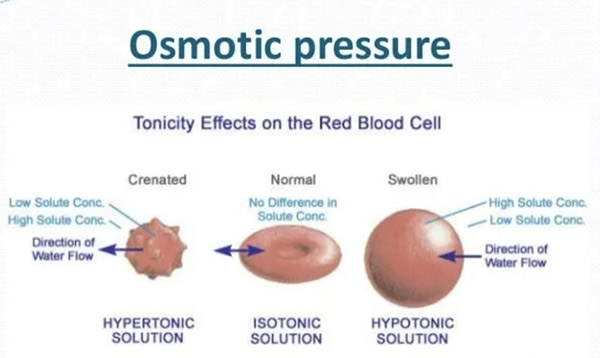
Interestingly, some of the lactate produced locally in your muscles actually diffuses into the bloodstream and crosses the blood brain barrier through monocarboxylate transporters (MCTs), and is utilized as fuel for the brain.60,61
Similar to ketones, lactate can be an important brain fuel, as at high lactate concentrations, up to 60% of the brain’s energy can come from lactate. Once the lactate reaches your brain — which occurs when you release the bands from your extremities — it increases brain derived neurotropic factor (BDNF),62 a member of brain growth factors that contributes to neuroplasticity and enhanced cognitive performance.63,64
I first learned about BFR in 2017 at a Beverly Hills biohacking event. I’ve been using it nearly every day for at least an hour a day since then and have compiled a number of insights in that time. The main difference between KAATSU and BFR is the tool you’re using. BFR can be done with restriction bands, but KAATSU uses a device that also provides intermittent and not just constant pressure.
From the start, I was really intrigued with the concept of BFR, so I purchased a KAATSU unit developed by Sato. In cycle mode, the KAATSU device produces 30 seconds of pressure followed by five seconds of no pressure, and does this for eight progressively increasing pressure steps per cycle. What this does is provide intermittent hypoxia, which catalyzes the steps I described above.
Since the intermittent hypoxia does not last more than 30 seconds, it does not damage your body, which can happen when you use the cheaper KAATSU bands or BRF bands that provide constant pressure. When I first started using BFR, I felt it was important enough to encourage people to get inexpensive bands that don’t provide intermittent compression. The BFR bands were only $15 and allowed far more people to access this powerful fitness strategy.
But after using the unit for five years, I have learned that when you use the cheap bands, or even KAATSU in the constant mode, you can get spastic large muscles. Using constant pressure increases the hypoxia time, and hence the lactic acid can build up to excessive levels. The only way to get the benefits without the downside is to avoid the constant mode and cheap bands.
This is why I only use the cycling KAATSU mode, not the constant mode or cheap bands, and why I now only recommend using the KAATSU intermittent hypoxia system, which is far safer and provides greater health benefits. Sato himself admits that the constant mode requires a lot of experience and know-how before it can be used safely. The cycling mode, however, is safe to use for anyone. “Even someone in their 80s can do the KAATSU cycle mode without risks,” he says.
 For a limited time, you can get 10% off the KAATSU band by using this link: www.kaatsu.com/go/NVIC
For a limited time, you can get 10% off the KAATSU band by using this link: www.kaatsu.com/go/NVICSince I first started using KAATSU, I have gained about 25 pounds of muscle mass after the age of 65, which is extraordinarily hard to do. I am convinced that this is largely a result of using KAATSU and getting enough protein, as I do not take any drugs at all, let alone performance-enhancing drugs.
If I had only done strength training without KAATSU, I would likely not have gained anywhere near as much muscle mass. Again, this is largely due to older people having decreased microcirculation to feed the Type 2 muscle stem cells. You need the VEGF to increase blood supply to these cells, and conventional strength training does not do that very well.
Why I Only Recommend KAATSU Cycle Mode
The KAATSU set is ideal as it is far easier to dial in to the correct pressures. You also get the benefit of intermittent pressure automatically, without having to adjust the bands yourself. With the KAATSU system you can control the tightness in two ways.
The initial tightness is after you manually tighten the bands. This is the base pressure and typically around 10 to 25 mm/Hg for the arms and 15 to 35 mm/Hg for the legs, depending on your age, vascular elasticity and physical condition.
The inflation pressure is what you set the compressor to pump the cuff up to. This ranges from 80 to 400 mm/Hg for both the arms and legs. KAATSU is the only unit that will cycle the inflation of the bands on and off, which, again, is far healthier for your muscles. Sato notes (see video above):
“I gradually increase the pressure on each set, personally, doing up to eight sets. The first set has no effect, but my muscles pump up significantly from the sixth to eighth sets. If you do that for two or three circuits, the KAATSU cycle mode will have the same effect as the KAATSU constant mode. Therefore, I recommend the KAATSU cycle mode to the general public.”
How to Determine Your Ideal Level of Resistance
Instead of using heavy weights that can increase your risk of injury during conventional strength training, BFR requires just 20% to 33% of the resistance used in conventional resistance training, which makes it much safer for everyone. This light weight is then combined with a high volume of repetitions while externally applied compression mildly restricts blood flow to the active skeletal muscles in the legs or arms.65
As for weight, your goal is to find the “sweet” spot. If you are elderly or have not been exercising regularly, this may mean no weights at all.
Ideally, you would have access to a variety of progressively increasing resistance movements to choose from, including body weight exercises. You typically won’t need to go higher than 25 pounds, though.
Once you have access to the weights, you can find the heaviest weight you can do for just one repetition of your planned exercise. This is your one-rep max (1RM).
Then you divide that weight by five (20%), four (25%) or three (33%). For example, if your max weight for a bicep curl is 25 pounds, you would select a 5-pound dumbbell to start.
If you don’t know your one-rep maximum, then it is always better to start too low, especially if this is your first time, as your tissues will need time to adapt to these pressures and movements. Eventually you will want to increase your weight so you notice the following signs during your KAATSU session.
|
Signs That You Are Using the Correct Weight
These two signs are an indication that you have activated your sympathetic nervous system by firing your Type II muscle fibers. This is because properly performed BFR is a high intensity exercise that will exhaust your Type I muscle fibers and activate your Type II fibers. You can also measure the circumference of your limb before and after the exercise. You should notice an increase of at least one-half inch and possibly 1 inch or more — or, alternatively, the muscle will most certainly feel tighter and appear more toned. Another great indication is that you will be able to do 30 reps the first set and then 15 to 20 reps the next and, most likely, are unable to do five to 10 reps in the last set because you are in muscle failure. It is important, though, not to fool yourself and stop just because it is hard. Muscle failure means that you are unable to do another rep if your life depended on it. |
Unless you are just starting (see warning box below), it is best to start by limiting your weight to only 20% of your 1RM and build up from there. By starting at a lighter weight, it will give your body a chance to adjust to BFR and avoid potential injuries.
An additional benefit is that if you stick with lighter weights you can train more frequently because you won’t cause as much muscle damage. For those interested in greater strength or muscle gains, you can increase to one-quarter and then to one-third the weight of your 1RM. If you are doing the exercises correctly, it will likely take you about three months to progress up to 33% of your 1RM. There is no need to go any higher than this.
If you don’t know your 1RM, then all you have to do is pick a weight you believe you can easily do 30 reps with and start there. If you can easily do all three sets at that weight, then it’s clearly too low a weight and you would benefit from increasing the resistance, especially if you don’t notice an increase of at least one-half inch in the circumference of your biceps after the exercise.
Conversely, if you are unable to complete 20 repetitions on your first set, the resistance is likely too high and needs to be decreased.
!WARNING FOR FIRST-TIME USERS
The only exception to these weight recommendations and initial pressure of the bands is when you are first starting out. It is important to realize that your tissues need time to adjust to BFR training. For the first session, you want to start with a light pressure, likely under 40%, and use only 10% of your 1RM. Then over the next two sessions increase to the minimum recommendations.
Important: You Need to Push Hard to Get the Benefits

It is important to recognize that the level of intensity you use is key. Muscle growth is highly dependent on metabolic factors, and training sets are ideally done to close to failure to achieve this.66,67
The number of repetitions completed during a training session is less important to cause long term changes in hypertrophy and strength than doing repetitions close to failure, which likely causes greater metabolic stress.
Perceived exertion is a major element here. You really need to push hard to muscle failure. This is a very subjective determination, but I hope the featured videos will give you an idea of the amount of intensity and effort one needs to put into this short exercise.
You can also notice if you are sweating and you are out of breath. Since BFR is a high intensity exercise and stimulates your sympathetic nervous system if done properly, this is precisely what you should be experiencing when you do BFR training.
A recent study in the elderly showed that physical weakness in aging may be due, at least in part, to impairments in brain and nerve function, rather than changes in the muscles themselves.68
The researchers did the study by asking participants to push to failure and once they said they had, they stimulated the muscle electrically and were still able to get the muscle to contract, which indicated that the muscle was not at full failure. In fact, in most cases the muscle was still able to contract about 25% more.
If you are unable to push close to failure, you will not receive the maximum benefits possible from BFR. Also, shorter recovery periods between exercises and sets will heighten the metabolic stimulus to enhance your body’s ability to build muscle and strength.69
Remember, you can start slowly and work your way up over time. Building muscle is a marathon, not a sprint. This is especially important if you are elderly or if you have been mostly sedentary; you likely will not need to use any weights.
You can start with just the weight of your body and gradually progress to 1- and 2-pound weights. But if you really are interested in triggering the benefits of reversing sarcopenia, then it is key to push hard — otherwise you will not achieve all the wonderful metabolic benefits that BFR has to offer you.
General KAATSU Workout Guidance
Although you can adapt KAATSU training to many types of resistance training, including machines, it seems the ideal way to implement it is by using simple dumbbells. Here’s some general workout guidance when using KAATSU.
Number of repetitions in each set:
|
Typically, upon starting KAATSU, you’ll notice a high perceived degree of difficulty. However, over a few weeks this perception of difficulty dampens as adaptation to training occurs.70 At that point, it becomes important to continue to push with the same level of intensity.
One of the major advantages of KAATSU versus high-load resistance training is that you cause far less muscle damage, which allows you to train more frequently. The frequency of training needs to be individualized as it varies widely. It can range from as little as twice a week up to three times a day, depending on your fitness and training goals.
Typically, the lower the percentage of 1RM used, the more frequently BFR can be done.71 Heart rate variability and the Oura ring can also be used to determine your ideal recovery periods. The Oura ring measures your heart rate all night and will tell you not only your lowest heart rate but also at what time it occurs. The higher your heart rate and the closer your lowest heart rate time is to awakening, the more recovery you need.
How to Perform BFR

Begin by applying the bands to your upper arm, very close to your armpit, just where your bicep muscle begins and deltoid muscle ends.
On your legs, you can apply them right below your hips at the top of your quads, close to your groin. There are misconceptions that you need to put the bands close to the muscle you are seeking to focus on, but this is unnecessary.
There is a crossover training effect and your muscles that aren’t blood flow-restricted will also receive benefit once the bands deflate. In other words, you will gain benefits in your chest muscles even though you are only restricting your arm muscles.
Do not put the bands over your knees or elbows, as this could cause nerve damage. Only put the bands on your body as described above, because the goal is to increase your vascular elasticity and elicit a metabolic and hormonal response that ultimately leads to aesthetic and muscular improvement.
When you engage in the exercise properly, lactic acid will accumulate in the muscle, which will be associated with a burning-like pain due to the excess hydrogen ions being produced. It will clearly be uncomfortable, especially as you push to muscle fatigue. It is important to understand that this subjective sensation of discomfort will improve with time.
It will likely take four to six weeks to develop the strength and hypertrophy gains. Once achieved, a study72 in elderly participants showed that doing BFR training twice a week was sufficient to maintain the gains. When training decreased to once a week, the gains failed to be maintained.
If you are able to, there is benefit to doing BFR every day. You can just vary the number of exercises you do per day. It could be as simple as applying the bands to your legs and walking for 30 minutes, or putting them on your arms and swimming. It doesn’t have to involve weights. You can also use them in your favorite sport.
Get Stronger and Healthier With Age
In summary, the four key lifestyle strategies that have allowed me to get healthier with age are TRE in combination with a cyclical ketogenic diet (which I’ll review below); exercising while fasting; and using KAATSU in my strength training routine.
While doing any one of these in isolation would likely improve your health, when done in combination, they really catalyze synergistic changes that optimize your entire system. The best news of all is that it’s never too late to start. My transformation began in my 50s, and I feel better now at 69 than I did back then. You can transform your health and physique too. You just have to get started, and keep going!
Your Food Choices Are Crucial for Muscle Building
My understanding of optimizing nutrition for health has been a greater than five-decade journey. At the beginning, I fell into the low-fat diet myth and thought I was eating healthy with my grains and margarine alternatives, but I was fooled. The key here is that I was motivated to make the right choices: I simply lacked proper mentoring and information.
That was one of the primary reasons I started this website over 25 years ago. I believed that people didn’t need to make the same silly mistakes I made, and by sharing my insight I could save them needless pain and grief.
Another major mistake I made was never taking time off from eating. It seemed to make sense that you need to eat around the clock, and that going without food for days could wreck your health by losing muscle mass from inadequate protein intake. After researching this, I realized it was seriously wrong and counterproductive. Your body actually requires regular intervals when you aren’t eating, and failing to do so is a prescription for metabolic disaster.
Research by Satchidananda Panda, Ph.D., suggests 90% of people eat across more than 12 hours per day, and perhaps 50% of the population eat across 16 hours a day. There are even many who wake up in the middle of the night to eat.
This constant hunger occurs because when you’re using carbs rather than fat as your primary fuel, you need constant refueling as carbs burn so much quicker than fat. I wrote extensively about this in my best-selling book, “Fat for Fuel,” Since your body has a minute supply of stored carbs relative to fats, you need to eat far more frequently to avoid feeling ravenously hungry and tired as your body runs out of fuel.
The remedy for this is twofold: Eat a cyclical ketogenic diet (high in healthy fats and low in carbs, with higher amounts of carbs cycled in), and restrict the window of time in which you consume all your meals each day. Both of these strategies will help retrain your body to burn fat for fuel, which is a sign of metabolic flexibility that is crucial for optimal health, and once you can burn fat, hunger is significantly decreased and you can go without food far longer.
Time-Restricted Eating Is a Key Health Principle
Time-restricted eating (TRE) is one of the most important health principles of our time. Contrary to modern belief, your body isn’t designed to be fed throughout the day, and the near-continuous grazing that most engage in can have serious health consequences.
When you eat throughout the day and never skip a meal, your body adapts to burning sugar as its primary fuel, resulting in the downregulation of enzymes that utilize and burn stored fat.73,74 As a result, you become progressively more insulin resistant and start gaining weight. Many biological repair and rejuvenation processes also take place while you’re fasting, and this is another reason why all-day grazing triggers diseases while fasting prevents them.75
Even though TRE was a major breakthrough for me, I have recently come to realize that it is not for everyone. However, it is for most people as 95% of the people in the U.S. are metabolically inflexible. If you are insulin resistant than TRE is vital to implement but only until you become insulin sensitive.
If you continue TRE for too long, you will create inflammatory stressors by having your body generate cortisol to have your liver create glucose because your body is low in it. So once you become metabolically flexible it is important to increase your eating window to eight to10 hours or even 12 hours in the summer.
There are a number of different intermittent fasting regimes, some of which are more extreme than others, but all are based on the premise that you need to fast for periods of time. TRE is one of the easiest to follow as you simply abstain from food for 16 to 18 hours a day and eat all your meals within a window of six to eight hours. A six- to eight-hour window seems to be close to the metabolic ideal for most.
You Must Eat Adequate Protein to Increase Muscle Mass
Your muscle is made of protein and if you don’t supply enough of the raw material your body will not be able to generate new muscle tissue. Fortunately, there is an easy formula to follow that will supply the crucial amino acids your body needs to stimulate muscle protein synthesis.
The most important amino acids will be the branched chain amino acids, leucine being the key. You will need about three grams of leucine within one to two hours of your workout to activate mTOR, the anabolic signal and trigger to build tissue.
If you eat enough high-quality protein according to the following formula you will invariably have enough leucine. The minimum is about 1.2 grams of protein per kilogram of body weight (0.54 grams/pound) per day. Athletes and the elderly could benefit from even higher levels at 1.6 grams/kilogram (0.71 grams/pound).
Just be sure to avoid seeds and all nuts (except macadamia) as they are high in omega-6 fat linoleic acid (LA), which will cause other metabolic complications. Chicken and pork should also be avoided as they are monogastric animals that are fed commercial grains that are high in LA. They also have high LA levels in their tissues.
- 1 von Haehling S, Morley JE, Anker SD. From muscle wasting to sarcopenia and myopenia: update 2012. J Cachexia Sarcopenia Muscle 2012;3:213–217
- 2 I.H. Rosenberg Sarcopenia: origins and clinical relevance J. Nutr., 127 (5 Suppl) (1997), pp. 990S-991S
- 3 Aversa Z, et al The clinical impact and biological mechanisms of skeletal muscle aging. Bone. 2019 May 22;127:26-36. doi: 10.1016/j.bone.2019.05.021
- 4 Calcif Tissue Int. 2018; 103(1): 35–43. doi: 10.1007/s00223-018-0388-2
- 5 J. Gerontol. A Biol. Sci. Med. Sci., 64 (12) (2009), pp. 1333-1336. doi: 10.1093/gerona/glp130
- 6 J. Am. Geriatr. Soc., 51 (3) (2003), pp. 314-322
- 7 J. Gerontol. A Biol. Sci. Med. Sci., 71 (1) (2016), pp. 63-71
- 8 Fed Pract 2017 Jul 9;34(7):24-32
- 9 Cell Metab 2009 Dec;10(6):507-515
- 10 Cell Metab10:507–515
- 11 Cell Biol. 2005 Oct;37(10):2047-63
- 12 Diabetes 1982 Nov; 31(11): 957-963
- 13 N Engl J Med 1990; 322:223-228
- 14 Diabetes Care 2009 Nov; 32(suppl 2): S157-S163
- 15 Int. J. Biochem. Cell Biol. 2005, 37, 2047–2063
- 16 J. Gerontol. A Biol. Sci. Med. Sci. August 2018; 73(8): 1070-1077
- 17, 27 Redox Biol. August 2020; 35:101513
- 18 Surg Today. 2018; 48: 151–157
- 19 Ageing Research Reviews 2018 Nov;47:123-132
- 20 Liver Transpl. 2013 Dec; 19(12): 10.1002/lt.23752
- 21 Clin. Cancer Res. 2009 Apr 15;15(8):2920-2926
- 22 Cancer 2014 Sep 15;120(18):2910-2918
- 23 Arch Gerontol Geriatr. May-Jun 2019;82:100-105
- 24 J Am Geriatr Soc. 2017; 65: e18–e22
- 25 Autoimmun Rev. 2018; 17: 518–529
- 26 EBioMedicine. 2019 Nov;49:381-388
- 28 Age Ageing. 2019; 48: 16–31
- 29 Ageing Research Reviews 2019 Dec;56:100960
- 30 J Physiol 2015;593:2721–2734, doi:10.1113/JP270343
- 31 Toda N. Age‐related changes in endothelial function and blood flow regulation. Pharmacol Ther 2012;133:159–176, doi:10.1016/j.pharmthera.2011.10.004
- 32 Life Sciences Volume 118, Issue 2, 24 November 2014, Pages 97-109 doi: 10.1016/j.lfs.2014.09.009
- 33 J Cachexia Sarcopenia Muscle 2016; doi:10.1002/jcsm12105
- 34 Am J Physiol Cell Physiol 2013;304:C717–28
- 35, 65 Frontiers in Physiology 2019; 10: 533
- 36 Sports Med. 45, 313–325. doi: 10.1007/s40279-014-0288-1
- 37 Eur J Appl Physiol Occup Physiol. 1998;77:189–191
- 38 Front Physiol. 2019; 10: 446, Discussion: Muscle Strength
- 39 J Appl Physiol. 1996;81(5):2004–12
- 40 Microvasc Res. 1968;1:1‐14
- 41 J. Strength Cond. Res. 26 611–617. 10.1519/JSC.0b013e3182281c69
- 42 Robergs, RA, et al. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol 287: R502–R516, 2004
- 43 Clin Physiol Funct Imaging. 2015 May;35(3):197-202. doi: 10.1111/cpf.1214
- 44 FASEB J. (2012) 26:3691–702. 10.1096/fj.11-203026
- 45 Ekmekcioglu C Nutrition and longevity – From mechanisms to uncertainties. Crit Rev Food Sci Nutr. 2019 Oct 21:1-20. doi: 10.1080/10408398.2019.1676698
- 46 Musaro, A, et al Counteracting sarcopenia: the role of IGF-1 isoforms Aging (Albany NY). 2019 Jun 15; 11(11): 3410–3411. doi: 10.18632/aging.102027
- 47 J Hum Kinet. 2018 Dec; 65: 249–26
- 48 Cell 2019 Mar 7;176(6):1248-1264
- 49 Med Sci Sports Exerc. 2012 Nov; 44(11): 2077–2083
- 50 J Physiol 2012; 590(17): 4351–4361, Myogenic stem cell content Page 4355 (PDF)
- 51 Journal of Applied Physiology May 1, 1999; 86(5): 1513–1518
- 52 Mol BiolCell. 2000;11(5):1859–97
- 53 J Appl Physiol.2012;113(2):199–205
- 54 Schoenfeld BJ. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. SportsMed. 2013;43(3):179–94
- 55 J Appl Physiol. 2010;108(6):1563–7
- 56 Med Hypotheses. 2012;78(1):151–4
- 57 J. Appl. Physiol. 2015;118:742–749. doi: 10.1152/japplphysiol.00054.2014
- 58 Nutrients. 2019 Apr; 11(4): 869. doi: 10.3390/nu11040869
- 59 Lang F, Busch GL, Ritter M, et al. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78(1):247–306
- 60 Ther. Adv. Psychopharmacol. 2017;7:85–89. doi: 10.1177/2045125316675579
- 61 Brooks G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018;27:757–785. doi: 10.1016/j.cmet.2018.03.008
- 62 Neurosci. Lett. 2011;488:234–237
- 63 Med. Hypotheses. 2017;106:1–5
- 64 Neurosci. Lett. 2016 Sep 6;630:247-253
- 66 Sports Medicine 2013;43:179–194
- 67 Frontiers in Physiology June 2019 (PDF)
- 68 JAMA Network Open September 25, 2019
- 69 Medical Hypotheses February 2015;84:145–149
- 70 J Sports Sci. 2019 Aug;37(16):1857-1864
- 71 Frontiers in Physiology 2019; 10: 533, BFR-RE
- 72 Proceedings of the International Conference on Sports and Exercise Science 2009. Bangkok, Thailand Pages 336-341 (PDF)
- 73 Cell February 8, 2018; 172(4): 731-743.E12
- 74 Medical News Today February 8, 2018
- 75 Science November 16, 2018; 362(6416): 770-775, Page 1
Can These Nutrients Help Prevent Muscle Wasting?
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2022/11/25/how-to-prevent-sarcopenia.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate.
Analysis by Dr. Joseph Mercola Fact Checked November 25, 2022<
STORY AT-A-GLANCE
- Age-related muscle loss, also called sarcopenia, begins at age 30 and affects at least half of the people over age 80; omega-3 fatty acids, the amino acid leucine, and the probiotic Lactobacillus paracasei PS23 help counteract sarcopenia
- Whey protein is high in leucine and has long been known as an excellent source of protein that is easily digested and absorbed. Leucine may regulate muscle protein turnover and is an effective way to optimize muscle growth when combined with resistance training
- Nicotinamide adenine dinucleotide (NAD+) is a substrate for important enzymes and impacts age-related amyloid protein aggregates in the muscle that affects muscle aging. Boost your levels through exercise, sauna bathing, fasting, and minimizing EMF exposure
- A Harvard study showed consuming protein is not enough to protect muscle mass, participants must also include strength training, which also improves basal blood flow in the lower extremities. This helps protect against functional impairment and metabolic syndrome
As you age, your body naturally tends to lose muscle. This condition is known as sarcopenia or muscle wasting. A 2022 study published in the journal Nutrients1 found increasing certain nutrients could lower your risk of sarcopenia as you age.
If you are not proactive, you can expect to lose approximately 15% of your muscle mass between your 30s and your 80s.2 An estimated3 10% to 25% of older adults under age 70 and half of those over age 80 have sarcopenia.
Even when you’re younger, if you are forced to stay in bed it can have a dramatic impact on your muscle mass. In one 2015 review,4 researchers found you could lose 5.2% of your muscle mass in the first two weeks of bed rest and by Day 23, you could have lost up to 10% of your quadriceps muscle mass. Strong muscles are required for mobility, balance and the ability to live independently.
Sarcopenia5 can increase the risk of falls and fractures, which ultimately can lead to hospitalization and surgery. It is important to know that sarcopenia is not related to your body mass. In other words, individuals who are obese can also lose muscle mass, which increases their risk for complications.
One meta-analysis6 of 35 studies and 58,404 people demonstrated the global prevalence of sarcopenia is 10% in men and women. Scientists understand the importance of muscle wasting as it relates to longevity and health. This led the Centers for Disease Control and Prevention7 to recognize it as an independently reportable medical condition.
When you have a reserve of muscle mass, it minimizes the challenges that result from muscle wasting8 if you become sick or hospitalized. Because muscle is lost far more easily and quickly than it’s built, it’s crucial to find ways to continuously promote and maintain muscle mass.
Nutritional Treatment Helps Counteract Sarcopenia
Researchers in the featured study9 knew that a variety of nutrients have shown effectiveness in supporting muscle. The randomized clinical trial was developed to analyze how effective two months of food high in omega-3 fats, leucine and probiotic Lactobacillus paracasei PS23 (LPPS23) would be on appendicular lean mass, inflammatory status, amino acid profile and muscle performance in people who were diagnosed with sarcopenia.
The researchers enrolled 60 participants who were within 4.8 years of 79.7 years and split them into an intervention or placebo group. The researchers prepared a customized diet schedule for both groups that provided 1.5 grams of protein per kilogram of body weight each day.10 Each group also received a dietary plan that consisted of approximately 30% lipids and 55% carbohydrates.
The researchers measured weight loss as compared to the individual’s weight history in the six months before their baseline visit. The subjects were questioned about how well they had followed their diet plan and physical activity recommendations. They also filled out a 24-hour dietary recall once a month.
The group that received the intervention took a supplement containing 500 mg of omega-3 fatty acid, 2.5 g of leucine and an LPPS23 probiotic. The control group received an isocaloric placebo. At the end of the study, the researchers measured body composition, physical performance, mood, blood pressure, muscle strength and functional status.
They concluded from the measurements that the intervention appeared to be a “valid strategy to counteract the progression of sarcopenia and sarcopenic-defining parameters in older adults.”11
Whey Protein Another Tool to Prevent Sarcopenia
As the featured study demonstrated, your diet plays a significant role in muscle development since your muscles need enough protein to stay viable. A 2011 paper12 published in the American Journal of Nutrition noted that the differences in digestion and absorption of dietary protein can modulate muscle growth.
So, while you need protein to build and maintain muscle, some proteins are more easily digested and absorbed than others. When you eat the right kind of protein it can make a difference in the potential risk for sarcopenia. The researchers in the featured study13 included the amino acid leucine in the intervention, which is also found in high concentrations in whey protein.14
Whey is a byproduct of cheese production and has long been acknowledged as an excellent source of protein. In the 2011 study, whey protein was compared to casein and casein hydrolysate, and was found to stimulate muscle protein growth the best, likely because of the leucine content.
One of the reasons that leucine is so important to prevent sarcopenia is that it helps regulate the turnover of protein in your muscle. A 1975 paper15 in the Journal of Clinical Investigation said leucine may also “play a pivotal role in the protein-sparing effect of amino acids.”
A more recent 2017 study16 explained the most effective way to optimize muscle building is to use a combination of resistance training followed by a protein meal, with leucine-rich whey being one of the most efficient proteins that can be used. However, a Harvard study demonstrated that simply taking leucine will likely be ineffective.17
Two groups of men over age 65 consumed either 0.8 grams of protein per kilo per day or 1.3 g of protein per kilo per day. The researchers found the high protein group did not experience an increase in lean muscle mass, or improve physical function or muscle strength, greater than the low protein group, most likely because they were not exercising.
Whey protein also contains glutathione, another important component in promoting and protecting muscle mass. It is thought to play an important role in muscle wasting, specifically in helping to modulate higher levels of oxidative stress18 often found in patients with sarcopenia. As noted in a 2012 review:19
“It has been suggested that oral antioxidant supplementation may contribute at reducing indices of oxidative stress both in animal and human models by reinforcing the natural endogenous defenses …
Antioxidants are substances able to inhibit the rate of oxidation. Mainly, antioxidant enzymes (e.g., catalase, superoxide dismutase (SOD), glutathione peroxidase, glutathione reductase) work to maintain a state of balance preventing the transformation of ROS and to convert them into more stable molecules (like water and molecular oxygen).”
NAD+ Combats Age-Related Muscle Deterioration
Studies have also proposed that mitochondrial dysfunction in the motor neurons may drive the development of sarcopenia. A 2021 paper20 published in Cell Reports compared the similarities between muscle aging and degenerative muscle diseases. The data revealed that protein aggregates deposit in skeletal muscle, which is a feature of muscle aging.
The researchers identified an amyloid-like protein that impairs mitochondrial function. While researchers have known that aggregated proteins could contribute to brain aging, this was the first time that data had shown it could contribute to muscle aging and directly damage the mitochondria.
The researchers first used a substance in worms and found it could reduce age-related amyloid protein aggregates.21 They found the same results in human muscle tissue from older subjects. They then went on to test nicotinamide adenine dinucleotide (NAD+) boosting nicotinamide riboside in aged mice and found it reduced the number and size of the amyloid aggregates within the skeletal muscle tissue.
NAD+ is a substrate for several important enzymes and is essential in metabolic processes, such as creating ATP in the mitochondria. A 2020 paper22 published in Endocrinology and Metabolism demonstrated that when the NAD+ salvage pathways in muscle are impaired, mitochondrial dysfunction and decreased muscle mass ensue.
If your NAD+ level is low, some simple lifestyle strategies can help. For example, exercise, fasting, minimizing electromagnetic field (EMF) exposure and sauna bathing can help improve your NAD+ levels. Exercise, heat exposure and fasting address low NAD+ because they are catabolic stressors that activate AMP protein kinase (AMPK).
This in turn activates an enzyme called NAMPT, which governs the NAD+ salvage pathway. Oxidative stress and inflammation can also deplete NAD+. Exercise, sauna and fasting can help reduce oxidative stress and inflammation, and as a result, less NAD+ is depleted.
The best way to increase NAD+ levels is to optimize your circadian rhythm by going to bed around 9 and getting up around 5 AM. The further you veer from these hours the more you challenge your circadian rhythm. It is also important to avoid blue light from screens and home lights after sunset and before sunrise.
Then you want to make sure you are doing regular strength training exercises. The best time to do them would be in the AM while you are fasting. You can also take niacinamide 50 mg powder three times a day. More is not better and will be counterproductive by inhibiting your longevity proteins (sirtuins). You can see my interview with Nichola Conlon for more details.
Strength Training Helps Preserve Muscle Mass and Heart Health
As the Harvard study23 demonstrated, consuming protein is not enough to protect your muscle mass. One study24 from the University of Michigan School of Public Health found those with lower muscle strength did not live as long as their peers with stronger muscles. After adjusting for confounding factors, the data continue to show those with low muscle strength had a 50% greater risk of dying early.
The data was pulled from a study of 8,326 men and women aged 65 and older. A loss of muscle in older adults may also be a primary driver of insulin resistance25 and declining strength may impact a reduction in daily physical activity, which also contributes to metabolic dysfunction.26
Researchers from the National Institute of Health and Nutrition in Tokyo, Japan,27 tested the hypothesis that a reduction in leg blood flow would be absent or minimal in people who regularly perform strength training exercises. They engaged a group of 104 men ages of 20 to 34 and 35 to 65 to compare whole-leg blood flow and vascular conductance between the groups.
The data showed no notable differences in the two age groups in those who used resistance training. However, there was a significant difference in the sedentary middle-aged group leading the team to conclude that a reduction in basal whole leg blood flow may be absent in men who routinely engage in resistance training.
The researchers suggested that resistance training could favorably influence leg perfusion. Lower levels of basal leg blood flow are associated with developing metabolic syndrome and functional impairment.28
I have been exercising for over 50 years. In the first 43 years, I exclusively used aerobic exercise. I didn’t realize that while it lowered the risk of heart disease, it is highly catabolic and eventually lowers the ability to build muscle. At the height of my running career, my upper arm circumference was 10.5 in.
However, contrast that with my arm circumference in December 2020 when it measured 15 inches. I stopped long-distance running and started resistance training. The results didn’t happen overnight, and I was well over 50 when I first began using resistance training.
The key to my success has been allowing time for significant recovery so the connective tissue and muscle can rebuild. I work with a trainer, but if you cannot afford a trainer, there are many great videos.
When you use resistance training and add the nutritional elements your body needs to grow muscles, you’ll reap the benefits. Remember to avoid doing the same exercise every day to allow the body to recover and repair so you get the benefits and avoid injuries.
- 1, 13 Nutrients, 2022;14(21)
- 2 Journals of Gerontology, 1995;50(5-8)
- 3 Journals of Gerontology, 2003; 58(10)
- 4 Extreme Physiology & Medicine 2015;4:16
- 5 Aging in Motion, What Is Sarcopenia?
- 6 Journal of Diabetes and Metabolic Disorders, 2017; 16
- 7 Federal Practitioner, 2017;34(7)
- 8 Current Opinions in Clinical Nutrition and Metabolic Care 2012; 15(1)
- 9 Nutrients, 2022;14(21) Abstract
- 10 Nutra-Ingredients, November 2, 2022
- 11 Nutrients, 2022;14(21) 5
- 12, 14 American Journal of Nutrition, 2011; 93(5): 997-1005
- 15 Journal of Clinical Investigation, 1975;56(5)
- 16 Journal of the International Society of Sports Nutrition, 2017, 43(14)
- 17, 23 JAMA Internal Medicine, 2018; 178(4)
- 18 Maturitas 2018;109
- 19 Journal of Aging Research, 2012;2012(316943)
- 20 Cell Reports, 2021;34(3)
- 21 Science Daily, January 20, 2021
- 22 Endocrinology and Metabolism, 2020;35(4) Section Mitochondrial dysfunction
- 24 University of Michigan School of Public Health, August 22, 2018
- 25 Diabetes Care, 2009;32(2)
- 26 Journals of Gerontology, 2018;73(8)
- 27, 28 Journal of Applied Physiology, October 1, 2005; doi.org/10.1152/japplphysiol.00061.2005
Why Collagen Is Crucial for Bones and Skin
Reproduced from original article:
https://articles.mercola.com/sites/articles/archive/2022/05/04/collagen-for-bones-and-skin.aspx
The original Mercola article may not remain on the original site, but I will endeavor to keep it on this site as long as I deem it to be appropriate, and will not be bullied into removing it.
Analysis by Dr. Joseph Mercola Fact Checked May 04, 2022

STORY AT-A-GLANCE
- Collagen is the most common and abundant of your body’s proteins. One of its primary purposes is to provide structural scaffolding for your various tissues to allow them to stretch while still maintaining tissue integrity
- Collagen is part of the secret of why tendons have the tensile strength of wire ropes and why healthy bones are so hard yet not brittle. As minerals are incorporated into the collagen, it cases the collagen fibrils to contract. This stress generates a mineral-collagen composite material composed of high-tensile fibers with properties reminiscent of reinforced concrete
- Loss of collagen is also one of the biggest contributors to visible signs of aging, such as wrinkles and dull or sagging skin. When your collagen level is high, your skin will tend to be soft, smooth and firm, because the collagen allows skin cells to repair and renew themselves continuously
- Collagen is crucial for connective tissues such as tendons, ligaments, cartilage and fascia, and these too tend to get weaker and less elastic with age. Connective tissue requires very specific raw materials in order to heal, namely animal-based collagen such as gelatin and bone broth
- Homemade bone broth using bones and connective tissue from grass fed, organically raised animals will produce the best result. If using a supplement, make sure it’s made from grass fed organic animals, such as beef bones. Collagen supplements made from cattle hides can be problematic, even if organic and grass fed
Collagen is the most common and abundant of your body’s proteins. One of its primary purposes is to provide structural scaffolding for your various tissues to allow them to stretch while still maintaining tissue integrity. While it’s commonly known that a collagen-rich diet can help counteract signs of aging in your skin, it’s also crucial for bone health,1,2,3 and this is less widely known.
Collagen’s Role in Bone
Bone is created as collagen fibrils mineralize together with carbonated hydroxyapatite (calcium apatite). Combined, they form a hybrid material that is very strong yet flexible.
What’s more, as other minerals (such as strontium- and calcium-based minerals) are deposited inside the collagen, it causes a reaction that triggers the collagen fibrils to contract. This stress generates a mineral-collagen composite material composed of high-tensile fibers with properties “reminiscent of … reinforced concrete,” to quote an April 2022 paper in the journal Science.4,5
In short, this explains why tendons have the tensile strength of wire ropes and why healthy bones are so hard yet not brittle. As explained by Phys.org, which reported the findings:6
“A team at the Max Planck Institute of Colloids and Interfaces (MPICI) has discovered new properties of collagen: During the intercalation of minerals in collagen fibers, a contraction tension is generated that is hundreds of times stronger than muscle strength …
This contraction of the fibers apparently occurs during mineral incorporation into the collagen, putting the mineral under enormous pressure, which increases the fracture strength of the composite …
The strength of bones is based on the structural interplay of soft, organic collagen fibers and the hard, crystalline mineral particles embedded in them, thus a hybrid material. The collagen gives the mineral particles an active pre-stress.
Civil engineers use a comparable mechanism in pre-stressed concrete with the aid of high-strength steel and thus produce crack-resistant structural elements.
‘It is also interesting from a medical or biological point of view to understand what happens in the process of mineralization in bones,’ says Dr. Wolfgang Wagermaier, group leader at the MPICI. He adds, ‘Many bone diseases are associated with changes in mineral content in bones and thus altered properties.’”
Collagen Can Help Improve Your Skin
Loss of collagen is also one of the biggest contributors to visible signs of aging, such as wrinkles and dull or sagging skin. When your collagen level is high, your skin will tend to be soft, smooth and firm, because the collagen allows skin cells to repair and renew themselves continuously.
By the time you reach your 80s, you have about four times less collagen than you did in your youth, which brings about the skin issues. A collagen-rich diet can go a long way toward slowing down these visible signs of aging.7,8,9 It also benefits your hair and nails.
That said, certain environmental and lifestyle factors can also have a negative impact on your collagen production, regardless of your age, making healthy, youthful skin hard to attain. Factors that can slow your body’s ability to manufacture collagen include:
| Hormone imbalances and thyroid dysfunction | Pollution and dust |
| Overwork | Hydrogenated cooking oils |
| Processed foods | Nutritional deficiencies |
| Fluoridated water | Radiation |
| Excessive sun exposure | Sugar |
| Stress | Poor liver or kidney function |
If you’re vegetarian, you may also have a more difficult time keeping up your collagen intake, because it’s stored in animal bones. It’s one of the reasons why bone broth is now considered a superfood.
When it comes to skin health, it’s important to realize that topically applied collagen cannot cross into deeper skin layers, so most collagen-containing skin creams are likely a waste of money. To really make a difference, you need to tackle the problem from the inside-out, making sure you’re getting enough collagen, either through collagen-rich foods or a supplement.
Collagen for Soft Tissue Injury and Repair
Collagen is, of course, also crucial for connective tissues such as tendons, ligaments, cartilage and fascia, and these too tend to get weaker and less elastic with age. Connective tissue injuries are also problematic due to the fact that there’s very little blood supply in connective tissue, which slows down recovery.
Collagen is high in amino acids such as glycine, proline and hydroxyproline, which are the building blocks for the matrix of connective tissue. Your body automatically takes collagen into stressed areas and places where it’s needed the most.
While a muscle injury is fairly easy to fix and recover from, connective tissue requires very specific raw materials in order to heal, namely animal-based collagen such as gelatin and bone broth.
Collagen is high in amino acids such as glycine,10 proline and hydroxyproline, which are the building blocks for the matrix of connective tissue. Interestingly, your body automatically takes collagen into stressed areas and places where it’s needed the most.
On a side note, collagen will not count toward your daily protein intake, because it’s very low in branched-chain amino acids (such as leucine, isoleucine and valine, found in meat), which are the primary amino acids that stimulate muscle anabolism and muscle building.
Other Health Benefits of Collagen
Health benefits provided by collagen supplementation, aside from what I’ve already mentioned, include:
| Deeper sleep and serotonin release due to its glycine content11 |
| Reduced joint pain and stiffness,12 including osteoarthritis pain13 |
| Improved gut health and digestion, thanks to the presence of glycine14 |
| Improved blood pressure and reduced cardiovascular damage15 |
| Improved glucose tolerance16 |
| Reduced inflammation and oxidative damage, as glycine inhibits the consumption of nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is used as a reductive reservoir of electrons to recharge antioxidants once they become oxidized |
Types of Collagen
While 28 different types of collagen have been scientifically identified, most supplements will contain one or more of just three of these, which are known simply as:17,18,19
- Type 1 — collagen found in skin/hide, tendon, scales and bones of cows, pigs, chicken and fish
- Type 2 — formed in cartilage and typically derived from poultry
- Type 3 — fibrous protein found in bone, tendon, cartilage and connective tissues of cows, pigs, chicken and fish
Types 1, 2 and 3 comprise 90% of the collagen in your body.20 As for the difference between collagen and gelatin: Collagen is the raw material; gelatin is what you get when you cook the collagen.21
Choose Your Collagen Source Wisely
Historically, traditional diets provided ample collagen in the form of broth made from boiled chicken feet or beef bones. These are by far your best alternatives. If you decide to use a collagen supplement, it’s important to know what to look for. Here are some general questions to ask when shopping around:
•Has it been hydrolyzed? — Collagen supplements can be either unhydrolyzed (undenatured) or hydrolyzed (denatured). In their natural, unhydrolyzed state, collagen molecules are poorly absorbed due to their large size. Hydrolyzation refers to a processing technique that breaks the molecules down into smaller fragments, thereby enhancing intestinal absorption.
For this reason, most collagen products are hydrolyzed. However, the processing that most collagen supplements undergo to become hydrolyzed can also result in questionable byproducts that are best avoided. I review some of these problems in the video above.
•Is it organic and/or grass fed certified? — Laboratory testing has revealed many popular collagen and bone broth products contain potentially hazardous contaminants typically associated with concentrated animal feeding operations (CAFOs), such as heavy metals,22,23 chemicals like butylparaben, and various veterinary drugs,24,25 including antibiotics.
To avoid contaminants, make sure your collagen supplement is certified “100% Organic” by the U.S. Department of Agriculture (USDA)26 or, better yet, certified grass fed by the American Grassfed Association (AGA), which has the most rigorous standards. This also applies to gelatin, commonly used in cooking and baking.
•What raw materials is it made from? — Nonorganic collagen is almost universally made from hydrolyzed cattle hides, not beef bones. When made from cattle hide, even organic certification becomes questionable, because hides, organic or not, are still scraps from the leather tannery industry and have undergone intense processing with harsh chemicals.
Raw, newly skinned hides arrive to the tannery on large pallets, where they can remain to rot for weeks before being processed. Even though they’re salted, they’re not entirely preserved and the stench is overwhelming. The tannery process itself typically involves an acid bath and processing with harsh chemicals such as sulfuric acid or chromium salts.
Hides with scars and imperfections are discarded once they’ve gone through this processing, and these castoffs are what are used to make bovine hide-based collagen supplements. The already processed scraps then undergo additional processing to dissolve the hide and release the collagen peptides. So, while the raw hide may have come from an organically raised, grass fed cow, after all that chemical processing, just how organic is the final product?
My personal preference is to use a less denatured (unhydrolyzed) grass fed organic collagen supplement made from beef bones (not hide). Unhydrolyzed products tend to have a more balanced amino acid profile, and grass fed beef bones will avoid most contaminants.
That said, I still believe the natural approach is best. Making homemade bone broth using bones and connective tissue from grass fed, organically raised animals isn’t very complicated and will produce the best results. If you prefer chicken broth, consider using organic chicken feet. The claws are particularly rich in collagen.27
- 1 Bone 2010 Mar;46(3):827-3
- 2 PLoS One 2014 Jun 13;9(6):e99920
- 3 J Agric Food Chem. 2010 Jan 27;58(2):835-41
- 4 Science April 7, 2022; 376(6589): 188-192
- 5 National Natural Science Foundation of China, Chinese Scholars and Cooperators Achieved Progress in Bioprocessing-inspired Fabrication
- 6 Phys.org April 8, 2022
- 7 Skin Pharmacology and Physiology 2014; 27: 47-55 (PDF)
- 8 Journal of Medical Nutrition & Nutraceuticals 2015; 4(1): 47-53
- 9 J Drugs Dermatol. 2019 Jan 1;18(1):9-16
- 10 Amino Acids January 2018;50(1):29-38
- 11 J Pharmacol Sci 2012; 118: 145 – 148 (PDF)
- 12 Curr Med Res Opin. 2008 May;24(5):1485-96
- 13 Curr Med Res Opin. 2006 November; 22(11):2221-32
- 14 Am J Physiol 1982 February;242(2):G85-8
- 15 J Med Food. 2010 Apr;13(2):399-405
- 16 J Med Food. 2016 Sep;19(9):836-43
- 17 Nutraingredients.com March 19, 2015
- 18 Charlotte’s Book, Collagen Supplements
- 19 Amino-collagen.com, Types of Collagen
- 20 Woundresearch.com, A Review of Collagen and Collagen-Based Wound Dressings
- 21 Paleo Leap, Collagen Versus Gelatin
- 22 Rodale’s Organic Life May 19, 2017
- 23 ConsumerLab, October 4, 2019
- 24 Consumer Wellness Center October 5, 2017
- 25 Bonebroth.news October 5, 2017
- 26 USDA.gov, USDA Organic
- 27 T Health, 10 Chicken Feet Health Benefits August 6, 2015